Match each compound with the appropriate spectrum. a. b. c. d. .CI OH Br ZHN
Question:
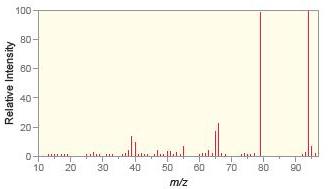
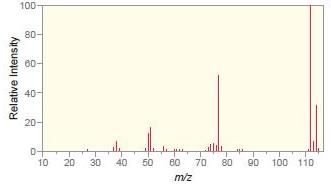
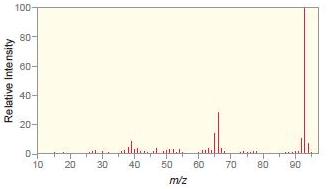
Match each compound with the appropriate spectrum.
a.
b.
c.
d.
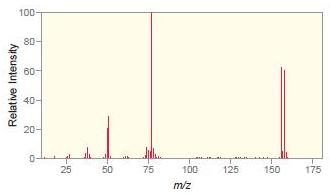
Transcribed Image Text:
.CI OH Br ZHN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
a ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Match each compound with the appropriate IR spectrum: a. b. c. d. e. f. `NH2 100- 60- 20- 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm-1) % Transmittance
-
Match the compound with the appropriate carbonyl IR absorption band: acyl chloride ........................ ~1800 and 1750 cm-1 acid anhydride ..................... ~1640 cm-1 ester...
-
Figures 12.15, 12.16, 12.17, and 12.18 are the IR spectra of four compounds: hexanoic acid, 1-pentanol, cyclohexane, and 3-pentanone, respectively. Match each compound with the correct spectrum,...
-
In Exercises 8194, begin by graphing the absolute value function, f(x) = |x| . Then use transformations of this graph to graph the given function. h(x) = x +31-2
-
What skills do project managers need now that they did not need in the past? What skills do you think project managers will need in the next 50 years that they do not have now? Provide a rationale...
-
In analyzing the motion of an automobile universal joint, the equation sec 2 A sin 2 Btan 2 A = sec 2 C is used. Show that this equation is true if tan A cos B = tan C.
-
Use the same information as presented in question (22) but assume that Arlington pays cash of $2.3 million. No stock is issued. An additional $40,000 is paid in direct combination costs. For each of...
-
IPO Pricing the Eyetech IPO was underpriced by about 54 percent. Should Eyetech be upset at Merrill Lynch over the under-pricing?
-
A flexible budget for 15,000 hours revealed variable manufacturing overhead of $90,000 and fixed manufacturing overhead of $120,000. The budget for 20,000 hours would reveal total overhead costs of:...
-
1. Create a time-phased assembly chart to determine when the 10 cars can be delivered. 2. What adjustments are needed in inventory levels, lead times, and batch sizes to fill an additional customer...
-
The mass spectrum of an unknown hydrocarbon exhibits an (M+1) peak that is 10% as tall as the molecular ion peak. Identify the number of carbon atoms in the unknown compound.
-
The sex attractant of the codling moth gives an IR spectrum with a broad signal between 3200 and 3600 cm -1 and two signals between 1600 and 1700 cm -1 . In the mass spectrum of this compound, the...
-
You are holding one of the tethers attached to the top of a giant character balloon that is floating approximately 20 feet above ground level. You are standing approximately 100 feet ahead of the...
-
Research a company that declared a 100% stock dividend or a two-for-one split Contrast the differences between a stock dividend and a stock split. Imagine that you are a stockholder in a company....
-
What are your ideas for Implementation and Assessing the Solution? How did you implement and assess the success? What should the time frame look like? What resources will be needed? What criteria...
-
What is an aesthetic question a viewer might ask about a work of art? 1 . What principles of design were used to make this work? 2 . What qualifies a functional object like this as a work of art? 3 ....
-
2. A phase diagram is shown below for an allotropic metal. Sketch and label possible Gibbs free energy curves for the 3 phases, as a function of temperature for the pressure indicated. Does the a or...
-
Recommend at least one (1) way a business with which you are familiar could use social media / buzz marketing to increase sales and awareness (e.g., social media awareness) of your...
-
Solve the equation. Check your answers. V5-x=3
-
Burberrys competitive advantage is through its differentiation strategy. What risk should Burberry remain aware of?
-
(a)Construct a hybrid orbital picture for the hydronium ion (H3O+) using oxygen sp3 hybrid orbitals. (b) How would you expect the H-O-H bond angles in hydronium ion to compare with those in water...
-
(a)Construct a hybrid orbital picture for the hydronium ion (H3O+) using oxygen sp3 hybrid orbitals. (b) How would you expect the H-O-H bond angles in hydronium ion to compare with those in water...
-
Which of the atoms in each of the following species has a complete octet? What is the formal charge on each? Assume all unshared valence electrons are shown. (a) CH (b) :NH3 (c) :CH3 (d) BH3 (e) :T:...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


