Methylmagnesium bromide reacts rapidly with ethylene oxide, it reacts slowly with oxetane, and it does not react
Question:
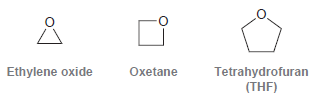
Explain this difference in reactivity.
Transcribed Image Text:
Oxetane Ethylene oxide Tetrahydrofuran (THF)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Ethylene oxide has a high degree of ring strain and readily functions as an electrophile in an S ...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write an equation for the reaction, if any, of p-bromobenzaldehyde with each of the following reagents, and name the organic product. a. Methylmagnesium bromide, then H3O+ b. Methylamine (CH3NH2) c....
-
A compound reacts with methylmagnesium bromide followed by acidification to form the product with the following 1H NMR spectrum. Identify the compound. 6 (ppm) 2 frequency
-
Ethylene oxide, C2H4O, decomposes when heated to give methane and carbon monoxide. C2H4O(g) CH4(g) + CO(g) The following kinetic data were observed for the reaction at 688 K: Find the rate law and...
-
DBU Systems manufactures testing equipment for the communications industry. In developing a new device for maritime communication, the design group has estimated the following unit costs. Metal...
-
Parent Co. purchases 100% of Son Company on January 1, 20X1 when Parent's retained earnings balance is $520,000 and Son's is $150,000. During 20X1, Son reports $15,000 of net income and declares...
-
What are some of the drawbacks to a strategy that attempts to capitalize on the first-mover advantage?
-
What are the three key factors in supplier partnerships? LO.1
-
The following amounts appeared on Davison Company's adjusted trial balance as of October 31, 2014, the end of its fiscal year: Required 1. Prepare a classified, multiple-step income statement for...
-
5015 (3 completely E10-27 (similar to) Snap Dragon Photo reported the following figures on its December 31, 2018, income statement and balance sheet: (Click the icon to view the figures.) Compute the...
-
1) Spin- system is known to be in an eigenstate of S.n with eignevalue h/2, where n is a unit vector lying in the xz-plane that makes an angle B with the positive Z-axis. a) Suppose that S, is...
-
The Williamson ether synthesis cannot be used to prepare tert-butyl phenyl ether. a. Explain why this method cannot be used in this case. b. Suggest an alternative method for preparing tert-butyl...
-
Identify the reagents necessary to accomplish each of the following transformations. >
-
Understand basic currency measures. LO,1
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
Suppose that two linear equations are graphed on the same set of coordinate axes. Sketch what the graph might look like if the system has the given description. (a) The system has a single solution....
-
Consider the circuit of Fig. 7.97. Find v0 (t) if i(0) = 2 A and v(t) = 0. 1 3 ett)
-
In step 6 of fatty-acid biosynthesis (Figure), acetoacetyl ACP is reduced stereo specifically by NADPH to yield an alcohol. Does hydride ion add to the Si face or the Re face of acetoacetylACP?...
-
In step 7 of fatty-acid biosynthesis (Figure), dehydration of a ?-hydroxy thioester occurs to give trans-crotonyl ACP. Is the dehydration a syn elimination or an anti elimination? CH3SCOA Acetyl CoA...
-
In step of fatty-acid biosynthesis (Figure), reduction of Trans-crotonyl ACP gives butyryl ACP. A hydride from NADPH adds to C3 of the crotonyl group from the Re face, and Protonation on C2 occurs on...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


