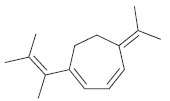
Predict the expected λ max of the following compound:
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
Base 217 Additional double bond...View the full answer

Answered By

Ayush Mishra
I am a certified online tutor, with more than 3 years of experience in online tutoring. My tutoring subjects include: Physics, Mathematics and Mechanical engineering. I have also been awarded as best tutor for year 2019 in my previous organisation. Being a Mechanical Engineer, I love to tell the application of the concepts of science and mathematics in the real world. This help students to develop interest and makes learning fun and easy. This in turn, automatically improves their grades in the subject. I teach students to get prepared for college entry level exam. I also use to teach undergraduate students and guide them through their career aim.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compound Y has molecular formula C 7 H 12 . Hydrogenation of compound Y produces methylcyclohexane. Treatment of compound Y with HBr in the presence of peroxides produces the following compound:...
-
Predict whether the following compound will be aromatic, nonaromatic, or antiaromatic. Explain your reasoning.
-
Use Woodward-Fieser rules to estimate the expected λmax for each of the following compounds: (a) (b) (c) (d)
-
The supplies account had a beginning balance of $1,523. Supplies purchased during the period totaled $4,928. At the end of the period before adjustment, $382 of supplies were hand. Prepare the...
-
A new highway to Siracha, Thailand, is to be constructed east of Bangkok, across a region of deep deposits of very soft marine clay. A typical soil profile is shown in Fig. 8.21(a). The average Cc =...
-
In Exercises use the Integral Test to determine the convergence or divergence of the p-series. 18 n=1 n 1 1/2
-
Val is in financial difficulty, and its stockholders and creditors have requested a statement of affairs for planning purposes. The following information is available: 1. The company estimates that...
-
Charles River Associates is considering whether to call either of the two perpetual bond issues the company currently has outstanding. If the bond is called, it will be refunded, that is, a new bond...
-
Adjust FVA at Year - End. On November 1 of Year 1 , Drucker Co . acquired the following investments in equity securities measured at FV - NI . Kelly Corporation 8 0 0 shares of common stock ( no -...
-
For each of the following, write the electron configuration and Lewis symbol: a. As b. As3+ c. Se d. Se2
-
Majority voting ensures that government will produce only those public goods for which benefits exceed costs. Why?
-
HQ Company is considering a 1-for-3 reverse stock split. HQs stock is currently selling for $3 per share. a. What will the price of the stock be after the stock split? b. HQ plans to pay a dividend...
-
A phone company charges $14.99 a month and 10 cents for every minute above 120 minutes. Write an expression for the monthly phone charge, P, in dollars, as a function of the number of minutes, m,...
-
1. Mainland purchased a machine for $85,000 on 1 January 20x7 and assigned it a useful life for 10 years. On 31 March 20x9 it was revalued to $93,000 with no change in useful life. Complete the table...
-
Find the equation of the regression line and identify a characteristic of the data that is ignored by the regression line X 10 8 13 9 11 14 6 4 12 7 5 Y 7.46 6.77 12.74 7.11 7.81 8.84 6.08 5.39 8.15...
-
For each of the following independent cases, fill in the missing amounts in the table: (Indicate the effect of each variance by selecting "F" for favorable, "U" for unfavorable.) Case Direct Labor...
-
All views expressed in this paper are those of the authors and do not necessarily represent the views of the Hellenic Observatory or the LSE George Alogoskoufis Greeces Sovereign Debt Crisis:...
-
Current Attempt in Progress Nash Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,812,000 on March 1, $1,212,000 on June 1,...
-
Problems 98106 are based on material learned earlier in the course. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for the final exam. The...
-
A supermarket chain is interested in exploring the relationship between the sales of its store-brand canned vegetables (y), the amount spent on promotion of the vegetables in local newspapers (x1)...
-
Which of the following substances would undergo the halo form reaction? (a) CH3COCH3 (b) Acetophenone (c) CH3CH2CHO (d) CH3CO2H (e) CH3C N
-
One way to determine the number of acidic hydrogens in a molecule is to treat the compound with NaOD in D20, isolate the product, and determine its molecular weight by mass spectrometry. For example,...
-
Base treatment of the following ?, ?-unsaturated carbonyl compound yields an anion by removal of H from the y carbon. Why is hydrogen?s on the y carbon atom acidic? LDA 1:0-I I.
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


