Predict the major product for each of the following reactions: a. b. c. d. e. f. g.
Question:
a.
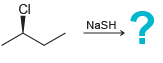
b.
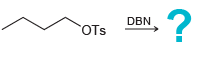
c.
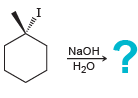
d.

e.
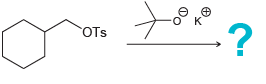
f.
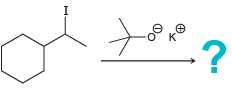
g.
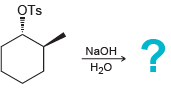
h.
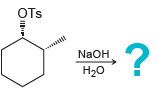
i.
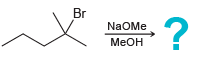
j.
Transcribed Image Text:
CI :? NaSH DBN OTs
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a b c...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product(s) and mechanism(s) for reaction between 2-bromobutane (sec-butyl bromide) and each of the reagents in Problem 42. (a) KCl in DMF (b) KI in DMF (c) KC1 in CH3NO2 (d) NH3 in...
-
Predict the major product(s) and mechanism(s) for reaction between 2-bromo-2-methylpropane (tert-butyl bromide) and each of the reagents in Problem 42. (a) KCl in DMF (b) KI in DMF (c) KC1 in CH3NO2...
-
Predict the major product(s) for each of the following reactions: 1) Hg(OAC)2, -0 2) NABH, ? - O, NaOH, cold Br2 H20 Pt
-
Use the data in Exercise 13.28.To familiarize yourself with recursive least squares, estimate the savings functions for 19701981, 19701985, 19701990, and 19701995. Comment on the stability of...
-
Read article "The Organization of Personality" by H. J. EYSENCK and write a reaction paper
-
In Exercises determine the location of a point (x, y, z) that satisfies the condition(s). xyz < 0
-
What is repetitive manufacturing? What are its advantages? What are its limitations? LO,1
-
Determine the amount of the standard deduction allowed for 2014 in the following independent situations. In each case, assume that the taxpayer is claimed as another person's dependent. a. Curtis,...
-
Tiger Inc. has two mos divisions. A Divisie podaces a technical component and its capacity is 10.000 units annually. Currently. A Divi s its product at a sale price of 519 per unit to external...
-
S. A. Harrington Company is a U.S.-based company that prepares its consolidated financial statements in accordance with U.S. GAAP . The company reported income in 2015 of $5,000,000 and stockholders'...
-
When 2-bromo-2-methylhexane is treated with sodium ethoxide in ethanol, the major product is 2-methyl-2-hexene. a) Draw the mechanism of this reaction. b) What is the rate equation of this reaction?...
-
What are the main arguments for and against the United States developing additional nuclear power plants to provide us with electricity over the next several decades? Which perspective do you find...
-
The truss is fabricated using members having a weight of 10 lb/ft. Remove the external forces from the truss, and determine the force in each member due to the weight of the members. State whether...
-
Given the following memory status below, compute how much does it cost to compact holes together with the following compaction strategies if 1 kbyte of movement costs 50 centavos. 0. OS OS OS OS OS...
-
ITG Pte Ltd ("ITG") is a company specialising in air-conditioner maintenance and servicing. It makes adjusting and closing entries every 31 December, which is the company's financial year-end. Unless...
-
(20 points) We know that when we have a graph with negative edge costs, Dijkstra's algo rithm is not guaranteed to work. (a) Does Dijkstra's algorithm ever work when some of the edge costs are...
-
Create a new user called cis605_usr. Use Master. assign a password of abcd, set check_policy to off and check_expiration to off (Why set these two to off?). Execute the sp_addsrvrolemember to add the...
-
Salmone Company reported the following purchases and sales of its only product. Salmone uses a periodic inventory system. Determine the cost assigned to the ending inventory using FIFO. Date Units...
-
In Exercises 23 through 28, compute the elasticity of demand for the given demand function D(p) and determine whether the demand is elastic, inelastic, or of unit elasticity at the indicated price p....
-
Using thermodynamic data from Appendix 4, calculate G at 258C for the process: 2SO 2 (g) + O 2 (g) 88n 2SO 3 (g) where all gases are at 1.00 atm pressure. Also calculate DG8 at 258C for this same...
-
Nitriles, RC N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propane nitrile, CH3CH2C N, if it has IR absorptions at...
-
The amount of energy required to spin-flip a nucleus depends both on the strength of the external magnetic field and on the nucleus. At a field strength of 4.7 1 rf energy of 200 MHz is required to...
-
Calculate the amount of energy required to spin-flip a proton in a spectrometer operating at 300MHz. Does increasing the spectrometer frequency from 200 to 300 MHz increase or decrease the amount of...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...

Study smarter with the SolutionInn App


