Predict the major product of the following reaction. HOS Dilute H2SO. ,5S. O,H
Question:
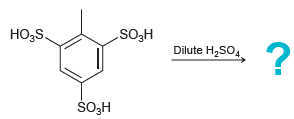
Transcribed Image Text:
H®OS Dilute H2SO. Но,5S. ŠO,H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
HOS S...View the full answer

Answered By

Ashok Kumar Malhotra
Chartered Accountant - Accounting and Management Accounting for 15 years.
QuickBooks Online - Certified ProAdvisor (Advance - QuickBooks Online for 3 years.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product of each reaction below: (a) (b) (c) 1) EtMgBr 2) H20 :? H. 1) PhMgBr 2) H20
-
Predict the major product of each reaction below: (a) (b) (c) RCO3H RCO, ? H RCO3H
-
Predict the major product for each E1 reaction: a. b. eat Br H2SO4 eat
-
Perform the indicated operations. Let k be a natural number. a. (-2) c. (-2)4 e. Is (-2)2+ positive or negative? b. (-2) d. (-2)5
-
How can an entrepreneur seeking funds to launch a business convince potential lenders and investors that a market for the product or service really does exist?
-
Solve for y in terms of x. log x y = ln e 3
-
3. A capital lease payable in the amount of $75,000 is noted in the internal debt records. This was recorded as an other financing source in the general fund.
-
What are the expected duration, variance, and standard deviation for an activity whose three time estimates are to = 2, tm = 14, and tp = 14?
-
A sole trader operates his business from a warehouse which has been damaged by a fire which occurred at the end of the financial year.After the fire the remaining inventory that is undamaged amounts...
-
Your brother has just started a new job as the Controller of an IESBA restricted audit client. You do not serve on the audit engagement. What steps must you take to ensure your independence is not...
-
It is found that K P is independent of T for a particular chemical reaction. What does this tell you about the reaction?
-
The following transformations cannot be accomplished, even with the help of blocking groups. In each case, explain why a blocking group will not help. (a) (b) . NO2 Br
-
Several companies are developing the manufacture and use of dimethyl ether, CH3OCH3, as an efficient and clean alternative to diesel fuel [Chemical and Engineering News, 1995 (May 29), 37-39]. Much...
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
a. Based upon the data in Exercise 20-7, determine the following: 1. Direct materials cost per equivalent unit. 2. Conversion cost per equivalent unit. 3. Cost of the beginning work in process...
-
A consultant is beginning work on three projects. The expected profits from these projects are $50,000, $72,000, and $40,000. The associated standard deviations are $10,000, $12,000, and $9,000....
-
The physical basis of some carbon monoxide detectors is the infrared detection of the unique stretching vibration of carbon monoxide at 2143 cm-1. How many times per second does this stretching...
-
The ==C-H stretching absorption of 2-methyl- I-pentene is observed at 3090 cm-1. If the hydrogen were replaced by deuterium, at what wavenumber would the ==C-D stretching absorption be observed?...
-
The ==C-H stretching absorption of 2-methyl- I-pentene is observed at 3090 cm-1. If the hydrogen were replaced by deuterium, at what wavenumber would the ==C-D stretching absorption be observed?...
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


