Question: Predict the major product(s) for each reaction below. (a) (b) (c) 1) LAH 2) , 1) PhMgBr 2) H,0
Predict the major product(s) for each reaction below.
(a)
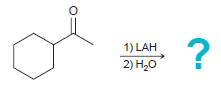
(b)
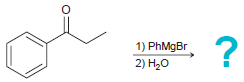
(c)
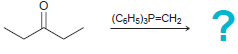
1) LAH 2) , 1) PhMgBr 2) H,0
Step by Step Solution
3.46 Rating (166 Votes )
There are 3 Steps involved in it
a b ... View full answer

Get step-by-step solutions from verified subject matter experts


