Treatment of catechol with formaldehyde in the presence of an acid catalyst produces a compound with molecular
Question:
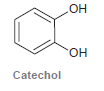
Transcribed Image Text:
HO он Catechol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
OH...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Hexachlorophene, a substance used in the manufacture of germicidal soaps, is prepared by reaction of 2, 4, 5-trichiorophenol with formaldehyde in the presence of concentrated sulfuric acid. Propose a...
-
Delrin (polyoxymethylene) is a tough self-lubricating polymer used in gear wheels. It is made by polymerizing formaldehyde in the presence of an acid catalyst. a. Propose a mechanism for formation of...
-
The industrial synthesis of methyl tert-butyl ether involves treatment of 2-methylpropene with methanol (CH3OH) in the presence of an acid catalyst, as shown in the following equation. CH3 H3C H3C...
-
A medical research study on a new medicine for multiple sclerosis is being conducted with 24 patients. After the study was concluded, it was determined that 16 patients reacted favorably to the...
-
Please discuss any special accounting treatments necessary to be aware of when disposing of part or all of an investment in a subsidiary, particularly address whether there are any affects of...
-
Describe the use of computer software in qualitative data analysis relative to each of the five qualitative approaches. Refer to the coding templates for each approach in your discussion. Complete a...
-
Web-based exercise. Each year, U.S. News & World Report ranks the nations colleges and universities. You can find the current rankings on the magazines Web site, www.usnews .com. Colleges often...
-
1) Suppose you were a project manager for Disney. Based on the information in this case, what critical success metrics do you think the company uses when designing a new ride; that is, how would you...
-
The Los Angeles Times headline announced today, Bellinger Angry His Deal Is Worse than Bettss. The L.A. Dodgers just signed Cody Bellinger to a contract consisting of eight, endofyear payments worth...
-
From the following T accounts of Brian?s Cleaning Service, (a) Foot and determine ending balances, (b) Prepare a trial balance in proper form for October 201X. Cash 111 Accounts Payable 211 Cleaning...
-
Devise an efficient synthesis for the following transformation (recall that aldehydes are more reactive than ketones): H.
-
Predict the major product(s) for each reaction below. (a) (b) (c) 1) LAH 2) , 1) PhMgBr 2) H,0
-
Show that z* 1 z* 2 = (z 1 z 2 )*.
-
1. Make a comparison between the leadership approaches "Trait Models" and "Behavioral Models". Discuss the main postulates and differences between the models and provide examples of a theory...
-
Applying the Central Limit Theorem: The amount of contaminants that are allowed in food products is determined by the FDA ( Food and Drug Administration ) . Common contaminants in cow milk include...
-
A collection of techniques used by social scientists to compile, summarize, and convey numerical data. Revised Research Question Hypothesis : Null Hypothesis : A null hypothesis, often known as H0,...
-
Adidas is an international sporting apparel/shoes brand. If Adidas was to enter a new foreign market, it would conduct a country market assessment. Identify the 4 components of the assessment....
-
Using the scenario linked in the Supporting Materials section, assume that you are the cost accountant for your company, and the CFO has asked for your analysis on purchasing materials from an online...
-
Solve each system by graphing. 6x - y = 2 X x - 2y = 4
-
Coastal Refining Company operates a refinery with a distillation capacity of 12,000 barrels per day. As a new member of Coastal's management team, you have been given the task of developing a...
-
Designate the (R) or (S) configuration at each chirality center in the following molecules. Cl SH Br Br
-
Albuterol, shown here, is a commonly prescribed asthma medication. For either enantiomer of albuterol, draw a three-dimensional formula using dashes and wedges for bonds that are not in the plane of...
-
(a) Write the structure of 2 ,2-dichlorobicyclo [2.2.1] heptane. (b) How many chirality centers does it contain? (c) How many stereoisomers are predicted by the 2n rule? (d) Only one pair of...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


