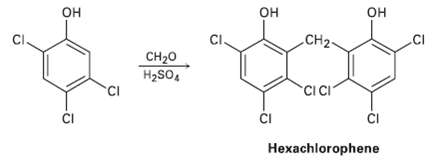
Hexachlorophene, a substance used in the manufacture of germicidal soaps, is prepared by reaction of 2, 4,
Question:
Hexachlorophene, a substance used in the manufacture of germicidal soaps, is prepared by reaction of 2, 4, 5-trichiorophenol with formaldehyde in the presence of concentrated sulfuric acid. Propose a mechanism for thereaction.
Transcribed Image Text:
н он он CI Он CI. CH2- сн20 H2SO, CIC CI ČI ČI Hexachlorophene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (18 reviews)
DR1 Formaldehyde is protonated to form a carbocation 2 0 H CI OH H CHOH OH Base OH The formal...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following reaction is important in the manufacture of sulfuric acid. At 900 K, 0.0216 mol of SO2 and 0.0148 mol of O2 are sealed in a 1.00-L reaction vessel. When equilibrium is reached, the...
-
A press produces parts used in the manufacture of large-screen plasma televisions. If the press is correctly adjusted, it produces parts with a scrap rate of 5%. If it is not adjusted correctly, it...
-
The strength of paper used in the manufacture of cardboard boxes (y) is related to the percentage of hardwood concentration in the original pulp (x). Under controlled conditions, a pilot plant...
-
R and Q form equal partnership RQ on February 15. Partner R has a tax year ending on July 31, whereas partner Qs tax year ends on October 31. What taxable year must the partnership use? a. February...
-
Determine the gross income of the beneficiaries in the following cases: a. Justin's employer was downsizing and offered employees an amount equal to one year's salary if the employee would...
-
Explain the differences between a CMO and a mortgage pass-through type of security?
-
On a balance sheet, what valuation must be reported for debt securities classified as available-for-sale? AppendixLO1
-
Determine whether the following statements about the nature of ethics are true or false. Explain your answers. a. Ethics is the study of why people act in certain ways. b. The solution to moral...
-
Entries for bond (held-to-maturity) investments The following bond investment transactions were completed by Starks Company: Jan. 31 Purchased 27, $1,000 government bonds at 100 plus accrued interest...
-
Write a Ruby or Bash script that will print usernames of all users on a Linux system together with their home directories. Here's some example output: gitlab:/home/gitlab nobody:/nonexistent . As you...
-
The compound MON-0585 is a nontoxic, biodegradable larvicide that is highly selective against mosquito larvae. Synthesize MON-0585 using either benzene or phenol as a source of the aromaticrings....
-
Benzenediazonium carboxylate decomposes when heated to yield N2, C02, and a reactive substance that can't be isolated. When Benzenediazonium carboxylate is heated in the presence of furan, the...
-
(Financial statements) Using the following codes, indicate which statement would be used to report each item for a not-for-profit hospital. BS Balance sheet SO Statement of operations 1. Land,...
-
Design a clocked D flip-flop, using a modified ECL circuit design, such that the output becomes valid on the negative-going edge of the clock signal.
-
An L2 steel strap having a thickness of 0.125 in. and a width of \(2 \mathrm{in}\). is bent into a circular arc of radius \(600 \mathrm{in}\). Determine the maximum bending stress in the strap.
-
Cars traveling from Canada to the United States through the Thousand Islands Border Crossing must stop for US Customs and Immigration. During the stop, each passenger in the car must present a...
-
Gasoline is pumped through a 2 in. sch 40 pipeline upward into an elevated storage tank at $60^{\circ} \mathrm{F}$. An orifice meter is mounted in a vertical section of the line, which uses a DP cell...
-
Change the recurring costs in Problem and Exercise 3 to $40,000 and redo the analysis. Problem and Exercise 3 Assume you are put in charge of launching a new website for a local nonprofit...
-
Do you think most managers in real life use a contingency approach to increase their leadership effectiveness? Explain.
-
General Electric Capital, a division of General Electric, uses long-term debt extensively. In a recent year, GE Capital issued $11 billion in long-term debt to investors, then within days filed legal...
-
N-butane is to be liquefied to make liquid petroleum gas (LPG). The butane is available at 25 C and 1 bar, it will be compressed to 15 bar in a compressor that has an isentropic efficiency of 85%,...
-
The oxymercuration reaction can be run in a methanol as the solvent rather than water. Predict the product of this reaction. 1) Hg(O,CCH)2, CH3OH 2) NaBH4, NAOH CH,CH,CH CH=CH;
-
The tautomerization of an enol to a ketone is catalyzed by either acid or base. In the acid-catalyzed mechanism H+ is added in the first step (see Figure). In the base-catalyzed mechanism, H+ is...
-
An unknown compound has the formula C6H10. (a) What is the DU for this compound? (b) When a solution of Br2 in CC14 is added to the unknown, the bromine color disappears. What information does this...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


