Benzenediazonium carboxylate decomposes when heated to yield N2, C02, and a reactive substance that can't be isolated.
Question:
Benzenediazonium carboxylate decomposes when heated to yield N2, C02, and a reactive substance that can't be isolated. When Benzenediazonium carboxylate is heated in the presence of furan, the following reaction is observed: What intermediate is involved in this reaction? Propose a mechanism for itsformation.
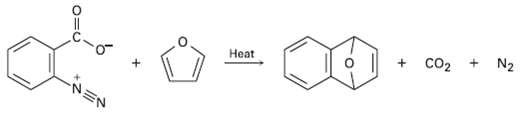
Transcribed Image Text:
+ Co2 + Heat N2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
0 NEN heat 0 ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
When ethyl 4-hydroxybutyrate is heated in the presence of a trace of a basic catalyst (sodium acetate), one of the products is a lactone. Propose a mechanism for formation of this lactone.
-
When the following compound is hydrated in the presence of acid, the unreacted alkene is found to have retained the deuterium atoms: What does the preceding statement tell you about the mechanism of...
-
The metal is heated in the presence of excess hydrogen, is it obvious which substance is the limiting reagent despite not specifying any quantity of reactant3 Given the statement the metal is heated...
-
Dale and Roy formed a partnership early this year. Dale contributed $150,000 cash in exchange for a 50% interest in the partnership. Roy contributed land with a tax basis of $90,000, and a fair...
-
Herbert was employed for the first six months of 2016 and earned $90,000 in salary. During the next six months, he collected $8,800 of unemployment compensation, borrowed $12,000 (using his personal...
-
Key figures for Apple and Google follow. Required 1. Compute the debt-to-equity ratios for Apple and Google for both the current year and the prior year. 2. Use the ratios you computed in part 1 to...
-
Under what circumstances are long-term investments in debt securities reported at cost and adjusted for amortization of any difference between cost and maturity value? AppendixLO1
-
Joey Cuono started his own consulting firm, Cuono Company SpA on June 1, 2017. The trial balance at June 30 is shown below. In addition to those accounts listed on the trial balance, the chart of...
-
Splish Inc. has negotiated the purchase of a new piece of automatic equipment at a price of $ 1 5 , 2 0 0 plus trade - in , f . o . b . factory. Splish Inc. paid $ 1 5 , 2 0 0 cash and traded in used...
-
Carissa Communications reported the following figures from its adjusted trial balance for its first year of business, which ended on July 31, 2016: Cash............... $4,100 Selling...
-
Hexachlorophene, a substance used in the manufacture of germicidal soaps, is prepared by reaction of 2, 4, 5-trichiorophenol with formaldehyde in the presence of concentrated sulfuric acid. Propose a...
-
Phenylboronic acid, C6H5B (OH)2, is nitrated to give 15% ortho-substitution product and 85% meta. Explain the meta-directing effect of the B (OH)2 group.
-
The reverse saturation current \(I_{o}\) of a silicon cell at \(40^{\circ} \mathrm{C}\) is \(1.8 \times 10^{-7} \mathrm{~A}\). The short circuit current when exposed to sunlight is \(5 \mathrm{~A}\)....
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
What do you think? Is it ethical for a leader to go undercover in his or her organization? Why or why not?
-
10m solution. If Ka(HA) = 10 then pOH of solution will be [Given : log4=0.6] (A) 6.7 (B) Greater than 6.7 & less than 7.0 (C) Greater 7.0 & less than 7.3 (D) Greater than 7.3
-
An Automobile engine can be modelled as an idealized four-stroke Otto cycle, although it actually consists of 6 steps: Step 0: A fuel-air mixture is drawn into a cylinder at constant pressure...
-
An unknown compound has the formula C7H12. (a) What is the DU for this compound? (b) The unknown reacts with H2 in the presence of Pd to give C7H16. What information dos these provide about the...
-
Explain the difference in the percentages of the products in these two hydroborationreactions: CH3 CH, QH CH, 1) BH3. THF 2) H,O,, NAOH CH,CH-CHCH-CH; + CH;CHCH,CHCH3 (43%) CH,CHCH=CHCH, (57%) 1)...
-
Explain why this reaction occurs with anti-Markovnikov regiochemistry: CI + CF,CH,CH CF;CH=CH2 + HCI
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


