Explain why this reaction occurs with anti-Markovnikov regiochemistry: CI + CF,CH,CH CF;CH=CH2 + HCI
Question:
Explain why this reaction occurs with anti-Markovnikov regiochemistry:
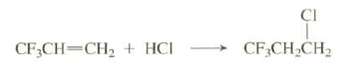
Transcribed Image Text:
CI + CF,CH,CH CF;CH=CH2 + HCI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
In this reaction the primary carbocation intermediate is ...View the full answer

Answered By

Sarah Khan
My core expertise are:
-_ Finance
-_ Business
-_ Management
-_ Marketing Management
-_ Financial Management
-_ Corporate Finance
-_ HRM etc...
I have 7+ years of experience as an online tutor. I have hands-on experience in handling:
-_ Academic Papers
-_ Research Paper
-_ Dissertation Paper
-_ Case study analysis
-_ Research Proposals
-_ Business Plan
-_ Complexed financial calculations in excel
-_ Home Work Assistance
-_ PPT
-_ Thesis Paper
-_ Capstone Papers
-_ Essay Writing etc...
5.00+
91+ Reviews
92+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain why the reaction shown in eq. 7.19 occurs much more easily than the reaction (That is, why is it necessary to protonate the alcohol before ionization can occur?) (CH) C-OH(CHCHO
-
Explain why hydroxide ion catalyzes the reaction of piperidine with 2,4-dinitroanisole, but has no effect on the reaction of piperidine with 1-chloro-2,4-dinitrobenzene. piperidine
-
Explain why nitration of quinoline (eq. 13.7) occurs mainly at C5 and C8. No 2 (13.7 0C 5-nitroquinoline NO2 8-nitroquinoline
-
MULTIPLE CHOICE: 6. The stage of production at which the individual jointproducts are identified is referred to as the: A. Split-off point B. Joint point C. Separate identification point D. Relative...
-
What is BI governance?
-
A company produces components for jet engines of commercial aircraft. One of its best-selling components is aluminum alloy housing. Because of the many different jet engines that its customers use...
-
A manufacturer of large-screen televisions wants to compare with a competitor the proportions of its best sets that need repair within 1 year. If it is desired to estimate the difference between...
-
Identify each of the following items as either part of taxable income or an exclusion, adjustment, or an allowable itemized deduction from taxable income for Brian Collins and Morgan Smithfield, a...
-
Which of the following statements is correct? Mike, who is a handy man, received concert tickets for doing repairs. The tickets had a FMV of $250 and a face value of $125. Mike included $125 in gross...
-
A microphone is attached to a spring that is suspended from the ceiling, as the drawing indicates. Directly below on the floor is a stationary 440-Hz source of sound. The microphone vibrates up and...
-
Explain the difference in the percentages of the products in these two hydroborationreactions: CH3 CH, QH CH, 1) BH3. THF 2) H,O,, NAOH CH,CH-CHCH-CH; + CH;CHCH,CHCH3 (43%) CH,CHCH=CHCH, (57%) 1)...
-
Explain why the hydration of this alkene occurs 1015 times faster than the hydration ofethene: OH H20 CH,CH,OCHCH3 CH.CH,OCH=CH, H,SO,
-
Wilkinson Co. is considering the following alternative financing plans. Income tax is estimated at 40% of income. Determine the earnings per share of common stock, assuming income before bond...
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
What is an effective exit interview system?
-
Suppose that fraction used = / 1.0 + 0.1Mt. for some parameter 1. Write the discrete-time dynamical system and solve for the equilibrium. Sketch a graph of the equilibrium as a function of ....
-
Write the equilibrium expression (K) for each of the following gas-phase reactions. a. N(g) + O(g)=2NO(g) b. NO(g)2NO(g) c. SiH4(g) + 2Cl(g) d. 2PBr3(g) + 3Cl(g) SiCl4(g) + 2H(g) 2PC13(g) + 3Br(g)
-
One of the steps in the biological pathway for carbohydrate metabolism is the conversion of fructose 1, 6-bisphosphatc into dihydroxyacetone phosphate and glyceraldehydes 3-phosphate. Propose a...
-
L-Fucose, one of the eight essential monosaccharide?s, is biosynthesized from GDP-D-mannose by the following three-step reaction sequence, where (H)P?guano sine diphosphate (a ribonucleoside...
-
Of the 19 L amino acids, 18 have the S configuration at the carbon. Cysteine is the only L amino acid that has an R configuration. Explain.
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


