Explain why the hydration of this alkene occurs 1015 times faster than the hydration ofethene: OH H20
Question:
Explain why the hydration of this alkene occurs 1015 times faster than the hydration ofethene:
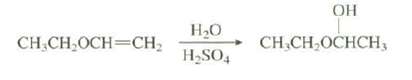
Transcribed Image Text:
OH H20 CH,CH,OCHCH3 CH.CH,OCH=CH, H,SO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (22 reviews)
The carbocation intermediate formed from this vinyl eth...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The alkene 3,3-dimethyl-1 -butene undergoes acid-catalyzed hydration with rearrangement. Use the mechanism of hydration and rearrangement to predict the structure of the hydration product of this...
-
Explain why compound A reacts faster than compound B when they undergo solvolysis in aqueous acetone. CH C-Cl CH3 CH CH
-
Why does a heavy parachutist fall faster than a lighter parachutist who wears a parachute of the same size?
-
The membrane filter technique is used to test a polluted water sample for coliform group. Three different dilutions of the water sample were prepared and each was filtered through 5 filter membranes....
-
Discuss the major issues in implementing BI. Discuss.
-
A consulting company would like to develop a method of predicting its total costs in a period. The following past costs have been recorded by the company in two periods: Number of client hours=...
-
Rat damage creates a large financial loss in the production of sugarcane. One aspect of the problem that has been investigated by the U.S. Department of Agriculture concerns the optimal place to...
-
Integrative-Investment decision: Holliday Manufacturing is considering the replacement of an existing machine. The new machine costs $1.2 million and requires installation costs of $150,000. The...
-
A project that has the following cash flow data. What is the project's IRR? a. 5.46% b. 6.89% c. 2.03% d. 6.09% e. 6.25%
-
5t2+3xy 2w2y dz 5. For z = and x = t2 + 1, y = vt? + 1 and w = et + 1, calculate the total derivative, dt dw at t = 0. Note: et dt
-
Explain why this reaction occurs with anti-Markovnikov regiochemistry: CI + CF,CH,CH CF;CH=CH2 + HCI
-
The addition of HCl to alkynes proceeds through a vinyl cation intermediate. Explain which of the two possible vinyl cations that could be formed from the addition of HCl to propyne is morestable....
-
You are in charge of allocating a $12,000 bonus to a team that recently met an important deadline. The team was in charge of designing a Web-based product for a client. The project lasted a year....
-
You have been employed as a systems analyst in the information systems organization of a medium-sized consumer goods manufacturer for three years. You are quite surprised when your manager offers you...
-
For your initial post, address the following: First, introduce yourself to the class by sharing a bit about yourself, such as your preferred name or pronouns, where you are from, what your major is,...
-
Question 8 : Consider the technology of Solar Panels. Which stage of the technology life cycle S curve is this technology in. Justify why ? Question 9 : The standard Product Life Cycle has 5 stages...
-
At Benihana restaurant a man wrenched his neck while ducking a piece of flying shrimp, requiring treatment by several doctors. By that summer, doctors determined surgery was necessary to treat...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
Should Henry have handled his meeting with Lucy any differently? How so? Henry is the maintenance manager at an older hotel that has been renovated in recent years. He is a newcomer to the property...
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
For a typical equilibrium problem, the value of K and the initial reaction conditions are given for a specific reaction, and you are asked to calculate the equilibrium concentrations. Many of these...
-
The amino acid threonine, (2S, 3R)-2-amino-3-hydroxybutanoic acid, has two chirality centers. (a) Draw a Fischer projection of threonine. (b) Draw a Fischer projection of a threonine diastereomer,...
-
Hemoglobin has pI = 6.8. Does hemoglobin have a net negative charge or net positive charge at pH = 5.3? At pH = 7.3?
-
Show how you could prepare the following -amino acids from the appropriate carboxylic acids: (a) Phenylalanine (b) Valine
-
Explain: An office building is renting for $10/sf, with 50,000 total leasable square feet. Office buildings in the area are selling for cap rates of 5.5%. What information do you have and what are...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...

Study smarter with the SolutionInn App


