Predict the product of the two-step procedure below, and draw a mechanism for its formation: 1) [H1,
Question:
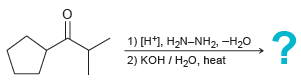
Transcribed Image Text:
1) [H1, Н-N-NH2 -H2о 2) КОН / Н-0, heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
The first two steps of this mechanism can be reversed first the amine attacks the carbonyl ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the product of the following reaction. O (1) BrMg (2) H2O MgBr (1 equiv.)
-
Draw a mechanism for the following reaction:
-
Draw a mechanism for the following transformation: 'CI Z Z
-
The ultimate test of fluency in MS and IR is whether you can determine a moderately complex structure from just the MS and the IR, with no additional information. The IR and MS of a compound are...
-
Decatur Company reports the following data: Sales...........................$526,500 Variable costs..................363,300 Fixed costs......................122,400 Determine Decatur Company's...
-
Provide a description of each tool or technology that provides sufficient information to make an informed decision on its potential adoption by the department. Areas to consider may include, but are...
-
Channel confl ict between manufacturers and retailers is likely to arise when manufacturers use ________________ websites.
-
The thing a lot of people dont understand about e-commerce is the degree to which it is a scale business, says Jeff Bezos, CEO of Amazon.com. Where a conventional retailer might have to double its...
-
Question 2: Traffic Control Systems makes bright rubber traffic cones. The company has two departments, Melting and Forming. Raw materials are introduced at various stages throughout the melting...
-
Audit standards distinguish auditors responsibility for planning procedures for detecting noncompliance with laws and regulations having a direct effect on financial statements versus planning...
-
Heyman and Ariely (2004) were interested in whether effort and willingness to help were affected by the form and amount of payment offered in return for effort. They predicted that when money was...
-
Propose a plausible mechanism for each of the following hydrolysis reactions: (a) (b) (c) (d) EtO OEt * * + 2 ELOH (b) N. * .N'
-
A compound of carbon, hydrogen, and oxygen was burned in oxygen, and 1.000 g of the compound produced 1.418 g CO2 and 0.871 g H2O. In another experiment, 0.1103 g of the compound was dissolved in...
-
What is a transistor, and what are its types?
-
Discuss the emerging role of nanotechnology in electrical engineering, focusing on its applications in enhancing electrical components like batteries, supercapacitors, and sensors.Explore the...
-
1. As resistors are added in parallel to an existing circuit, what happens to the voltage drop measured across each resistor? 2. In the circuit shown on the right, which path (left or right) will...
-
Solve each system using the elimination method. If a system is inconsistent or has dependent equations, say so. 1 -x + x - 1 6 1 2 y = -9
-
The MIT Sloan School of Management is one of the leading business schools in the U.S. The following table contains the tuition data for the masters program in the Sloan School of Management. a. Use...
-
State whether you would expect the entropy change, So, to be positive, negative, or approximately zero for each of the following reactions. (Assume the reactions take place in the gas phase.) (a) A +...
-
(a) What is the value of Go for a reaction where Keq = 1? (b) Where Keq = 10? (The change in Go required to produce a 10-fold increase in the equilibrium constant is a useful term to remember.) (c)...
-
Draw contributing resonance structures and a hybrid resonance structure that explain two related facts: the carbon-oxygen bond distances in the acetate ion are the same, and the oxygen's of the...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


