Predict the product(s) for each of the following reactions: (a) (b) (c) OH HNO, H,SO, OMe Br
Question:
(a)
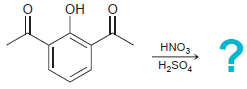
(b)
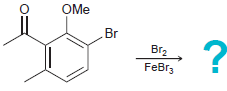
(c)
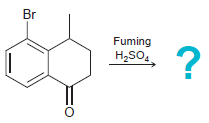
Transcribed Image Text:
OH HNO, H,SO, OMe Br Br2 FeBr3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (18 reviews)
a b c...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Find the area of the equilateral triangle of a side of 5 using the Babylonian way. A. 10;1,20 . 10:56,15 . 10:26,15 D. 10:25 A B C
-
The enthalpy change for each of the following reactions was calculated using bond energies. The bond energies of XO, YO, and ZO are all equal. XX + O=O XOOX; H = 275 kJ YY + O=O YOOY; H = +275 kJ...
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) (b) (c) (d) ...
-
For Example, 10.3 with = 0, verify that the stresses from equation (10.5.18) reduce to those previously given in Eq. (8.4.69). Data from example 10.3 Equation 10.5.18 Equation 8.4.69 Consider next...
-
Describe the common methods of establishing an advertising budget. Which method is most often used? Which technique is most often recommended? Why? Identify four basic methods for preparing an...
-
The acceleration g (in m/s 2 ) produced by the gravitational force of Earth on a spacecraft is given by g = 3.99 1014 /r 2 , where r is the distance from the center of Earth to the spacecraft. On...
-
In 2017, Tedfreds total fund balance increased by: a $3,000,000 b $2,500,000 c $1,500,000 d $1,000,000
-
On 1 January 2009 Henry Ltd issued a convertible debenture for 200 million carrying a coupon interest rate of 5%. The debenture is convertible at the option of the holders into 10 ordinary shares for...
-
For your final project you will be creating an Excel workbook and an Access database using your own data. You will be taking skills learned from each of the labs and applying them to each of the...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
For each compound below, identify which position(s) is/are most likely to undergo an electrophilic aromatic substitution reaction. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH3 NO2 O,N. CH3 O,N NO2
-
Calculate K P at 600.K for the reaction N 2 O 4 (l) 2NO 2 (g) assuming that H o R is constant over the interval 298 725 K.
-
Assume that $15,000,000 in floaters and $5,000,000 in inverse floaters are issued. How does this change the returns for the inverse floater when LIBOR is 2 percent, 4 percent, and 6 percent?
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
In Exercises 126129, determine whether each statement is true or false. If the statement is false, make the necessary change(s) to produce a true statement. The graph of a rational function can have...
-
The overall reaction and equilibrium constant value for a hydrogenoxygen fuel cell at 298 K is 2H 2 (g) + O 2 (g) 2H 2 O(l) K = 1.28 10 83 a. Calculate E cell and G 8 at 298 K for the fuel cell...
-
Show the elimination reactions that account for each of the following fragments in the CI mass spectrum of di-sec-butyl ether (Fig. 12.17b). (Fig. 12.17b) m/z =115 100 75 base peak131 80 73 60 101 40...
-
Show the elimination reactions that account for each of the following fragments in the CI mass spectrum of di-sec-butyl ether (Fig. 12.17b). (Fig. 12.17b) m/z =115 100 75 base peak131 80 73 60 101 40...
-
List two factors that determine the intensity of an infrared absorption.
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


