Propose a mechanism for the following transformation: 1) Excess LAH 2) H20 CH3
Question:
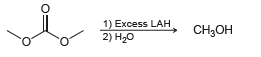
Transcribed Image Text:
1) Excess LAH 2) H20 CH3он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
JA HAH H ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a mechanism for the following transformation: NaOH, heat
-
Draw a mechanism for the following transformation: 'CI Z Z
-
Draw a mechanism for the following transformation: . HCI -
-
(2) Use Figure 10.2 shows a multi-degree-of-freedom (MDOF) model of three connected masses. Find the following for the MDOF system in Figure 10.2: Find expressions for the kinetic energy (T) and...
-
Quicksilver Delivery Service contracts to deliver Pete's Pizza Parlor's products to its customers for $5,000, payable in advance. Pete's pays the money, but Quicksilver fails to perform. Can Pete's...
-
Show that the expansion of (1 + x) 1/2 is not valid when x = 3.
-
1 To what extent was the doctor right to put so much emphasis on nonverbal communication during the consultation?
-
The data in Table 9E.1 represent individual observations on molecular weight taken hourly from a chemical process. The target value of molecular weight is 1,050 and the process standard deviation is...
-
Tamarisk, Inc. sells three different categories of tools (small, medium and large). The cost and net realizable value of its inventory of tools are as follows. Small Medium Cost $ 65,940 269.290...
-
Prepare journal entries for the purchase of a grader for $375,000: Purchased from the General Operating Fund. Purchased from the Equipment Reserve Paid by the General Operating Cash Account but...
-
Show the reagents you would use to achieve the following transformation:
-
A carbocation is resonance stabilized when it is adjacent to an oxygen atom: Such a carbocation is even more stable than a tertiary carbocation. Using this information, propose a mechanism for the...
-
Assume that Derra Foods, in the preceding problem, reported earnings before interest and taxes (with operating leases expensed) of $200 million. Estimate the adjusted operating income, assuming that...
-
The following information summarizes the activities in the Mixing Department for the month of March. Beginning inventory 1 , 0 0 0 units, 8 0 % complete Started and completed 2 4 , 5 0 0 units Ending...
-
What is your recommendation for the maximum size of coarse aggregate for the following situation? A continuously reinforced concrete pavement cross section contains a layer of No. 6 reinforced bars...
-
On January 1, 2024, Winn Heat Transfer leased office space under a three-year operating lease agreement. The arrangement specified three annual lease payments of $72,000 each, beginning December 31,...
-
A closed square pyramid tank (base width: 6.0 m; height 3.0 m), sitting on its square base, has a 1.0 m depth of water. Suppose this tank is inverted (turned upside down) and is made to stand on its...
-
P.4.3 Apply a Taylor series expansion to a mixed backward formula for the first derivative: (Ux)i = 1 Ax (aui-2+ bui-1 + cu + dui+1) Derive the family of second order accurate formulas and the...
-
For several years, one of Prestland Companys grocery stores has been unprofitable. At this time, management has not decided whether to close that location or do a major renovation effort and keep the...
-
Express these numbers in standard notation. a. 2.87 10-8 b. 1.78 1011 c. 1.381 10-23
-
Isoborneol (Problem 27.37) is converted into camphene on treatment with dilute sulfuric acid. Propose a mechanism for the reaction, which involves a carbocationrearrangement. C . H H2SO4 HO . H3...
-
Digit oxigenin is a heart stimulant obtained from the purple foxglove Digitalis purpurea and used in the treatment of heart disease. Draw the three-dimensional conformation of digitoxigenin, and...
-
What product would you obtain by reduction of digitoxigenin (Problem 27.39) with LiAlH4? By oxidation with pyridinium chlorochromate
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


