Rank the following compounds in order of increasing reactivity toward electrophilic aromatic substitution: Br Br- Br
Question:
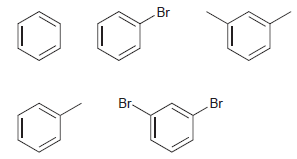
Transcribed Image Text:
Br Br- Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
Br Increasing ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following compounds in order of increasing reactivity toward HNO3 in H2SO4. (The references to equations will assist you with nomenclature.) (a) Chlorobenzene, benzene, nitrobenzene (b)...
-
Rank the following compounds in order of increasing reactivity in bromination. In each case, indicate whether the principal monobromination products will be the ortho and para isomers or the meta...
-
Rank the following compounds in order of increasing reactivity (least reactive first) in an SN1 solvolysis reaction in aqueous acetone. Explain your answers. (The structure of tert-cumyl chloride is...
-
When Ralph Lauren makes shirts to a customers exact preferences, what utility is provided?
-
What advantages does an LLC offer over an S corporation? Over a sole proprietorship? (LO 6: Describe the features of the LLC)
-
The time t (in s) to chemically change 5 kg of a certain substance into another is given by where x is the number of kilograms that have been changed at any time. Sketch the graph. 1 = 5 log (5 5x). t
-
Speed Limit A county is considering raising the speed limit on a road because they claim that the mean speed of vehicles is greater than 45 miles per hour. A random sample of 25 vehicles has a mean...
-
Sleepy Hollows General Fund budget for fiscal 2013 is based on the following estimated revenues and appropriations. Is Sleepy Hollow projecting a budgetary surplus or a deficit for 2013? Prepare the...
-
very important question kindly send me answer as soon as possible. Question 1 (22 marks) The following data has been gathered for Willow Ltd. for the month ended April 30, 2020: 1. The bank statement...
-
Shafer Company has gathered data on its overhead activities and associated costs for the past 10 months. Theodore, a member of the controllers department, has convinced management that overhead costs...
-
Under what conditions is K x > K P ?
-
A hard-working horse can lift a 350. lb. weight 100. ft. in one minute. Assuming the horse generates energy to accomplish this work by metabolizing glucose: C 6 H 12 O 6 (s) + 6O 2 (g) 6CO 2 (g) +...
-
Water is pumped into a spherical tank of radius 2 m from a source located 1 m below a hole at the bottom (Figure 5). The density of water is 1000 kg/m 3 . Calculate the work F(h) required to fill the...
-
2. (10 points) Two suppliers of products are available to supply the needs of four supermarkets. Each supplier can provide 90 units per day. Each supermarket would like to receive 60 units per day....
-
QUESTION 3 (11 marks) Midrand Ltd acquired a 90% interest in Bramely Ltd on 2 December 20.21 for R2 million. The consideration was settled as follows: Cash payment, Issue of 100 000 shares to the...
-
1. Prepare a Proforma Income Statement for ACCO 295 Corp. (30 points) Use the same Excel table provided to do the calculations with the class explanation. 1. Selling and administrative expenses were...
-
Sandy Foot Hospital expanded their cardiovascular unit to include more operating rooms. They negotiated a 20-year loan with monthly payments and a large sum of $250,000 due at the end of the loan....
-
Oscillations and Resonance Name Lab Procedure Answer questions in red. Download and run the HTMLS application \"resonance\". Driving force: 30 N Driving equency: 5 rad. '5 Spring constant: 5 - 'Irn...
-
In Exercises 135138, determine whether each statement makes sense or does not make sense, and explain your reasoning. I can evaluate some common logarithms without having to use a calculator.
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
How many different absorptions are observed in the spectrum of each of the following compounds? (a) (b) H,C CH3 - (CH,),C CH
-
How many different absorptions are observed in the spectrum of each of the following compounds? (a) (b) H,C CH3 - (CH,),C CH
-
Draw a Lewis structure for each of the following alkynes. (a) isopropylacetylene (b) cyclononyne (c) 4-methyl-1-pentyne
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


