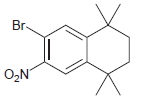
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each
Question:
(a)
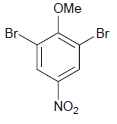
(b)
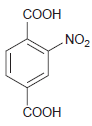
(c)
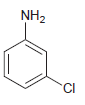
(d)
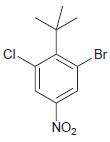
(e)
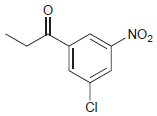
(f)
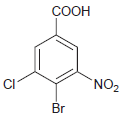
(g)
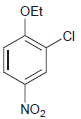
(h)
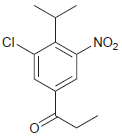
(i)
Transcribed Image Text:
OMe Br Br- NO2 СООН „NO2 СООН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a b c d e f g h ...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Provide a response to the questions within the case study. Responses to your classmates should include alternatives to their interpretations. Consider this as an email exchange with project teammates...
-
Outline a synthesis for each of the following compounds from the indicated starting material and any other reagents. (a) l-chloro-3, 5-dinitrobenzene from benzene (b) 2-chloro-4,6-dinitrophenol from...
-
Outline a synthesis for each of the following compounds from the indicated starting material and any other reagents. (a) (b) (c) ethylcyclopentane from cyclopentylethylene. CH2CO,H from CH CH...
-
Apply the product rule for exponents, if possible. y 4 y 5 y 6
-
Discuss a strategic plan for implementation and explain the expected outcomes
-
Prentiss plc operates a defined benefit pension plan and prepares financial statements to 31 March each year. The financial statements for the year to 31 March 2023 showed that the present value of...
-
13. Repeat the previous problem assuming that the stock pays a continuous dividend of 8% per year (continuously compounded). Calculate the prices of the American and European puts and calls. Which...
-
Figure employs a convention often used in circuit diagrams. The battery (or other power supply) is not shown explicitly. It is understood that the point at the top, labeled 36.0 V, is connected to...
-
Cullumber Company produces golf discs which it normally sells to retailers for $ 7 each. The cost of manufacturing 1 7 , 8 0 0 golf discs is: \ table [ [ Materials , $ 8 , 3 6 6
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Aromatic heterocycles are also capable of undergoing electrophilic aromatic substitution. For example, when furan is treated with an electrophile, an electrophilic aromatic substitution reaction...
-
When benzene is treated with methyl chloride and aluminum trichloride under conditions that favor trialkylation, one major product is obtained. Draw this product, and provide an IUPAC name.
-
Describe just-in-time inventory management.
-
+ Given f(x) = x - 9 and g(x) = x+9, complete the following. (a) Find f(g(x)) and g(f(x)). (Simplify your answers completely.) f(g(x)) = g(f(x)) = (b) What does this tell us about the relationship...
-
Case Study - Rhonda Rhonda is a 28-year-old woman who has been referred to your agency by a local probation officer. Rhonda reported that she has "fired" three counselors in the past and most...
-
Calculating depreciationpartial periods LO2, 3 West Coast Tours runs boat tours along the west coast of British Columbia. On March 5, 2020, it purchased, with cash, a cruising boat for $936,000,...
-
Question 1. Write down the form of partial fractions needed to decompose the following: 482+2 (a) s32s24s 482+2 (c) s36s20 482 +2 - 4s8 (b) 8. 3 - 282 482+2 (d) s3 +2s2 - 2 Note: You are not being...
-
On December 31, 2022, Ace Hardware reported the following information on its balance sheet Accounts Receivable Allowance for Doubtful Accounts $900,000 $54,000 (credit) During 2023, the Company had...
-
Determine how the value of f(x) changes when x triples and when x is reduced by half. Assume that x is a positive number. f(x) 3 X
-
What are multinational corporations (MNCs) and what economic roles do they play?
-
The standard free-energy difference between the two chair conformations of isopropylcyclohexane is 9.2 kJ mol-1 (2.2 kcal mol-1;. What is the ratio of concentrations of the two confirmations at 25C?
-
Which of the following alcohols can be synthesizecl relatively free of constitutional isomers and diastereomers by oxymercuration-reduction? Explain. H,C CH,CH2CH CH2CH CH H,C H,C
-
For each of the following reactions. provide the following information. (a) Give the structures of all products (including stereoisomers). (b) If more than one product is formed, give the...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


