Aromatic heterocycles are also capable of undergoing electrophilic aromatic substitution. For example, when furan is treated with
Question:
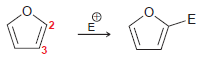
Transcribed Image Text:
12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
Attack at the C2 position proceeds via an intermediate with three resonance structures In contrast ...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
One ring of phenyl benzoate undergoes electrophilic aromatic substitution much more readily than the other. (a) Which one is it? (b) Explain your answer.
-
One of the following compounds undergoes electrophilic aromatic substitution predominantly at C-3, and one undergoes electrophilic aromatic substitution predominantly at C-4. Which is which? N CCH-CH...
-
Benzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as a. What are the major and minor products of the following reaction? b. Would...
-
Give the numerical coefficient and the degree of each term. 2
-
"Financing S & S Air's Expansion Plans with a Bond Issue", as explained in the case: 1. For each of the ten bond features listed, briefly describe the likely impact of each of the features on the...
-
Quartile sells jewellery through stores in retail shopping centres throughout the country. Over the last two years it has experienced declining profitability and is wondering if this is related to...
-
12. Let S = $100, K = $95, r = 8% (continuously compounded), = 30%, = 0, T = 1 year, and n = 3. a. Verify that the binomial option price for an American call option is $18.283. Verify that there is...
-
Comment on the following statement: More information is always preferred to less; you can never have too much information.
-
Required information [The following information applies to the questions displayed below.] Lacy is a single taxpayer. In 2020, her taxable income is $42,000. What is her tax liability in each of the...
-
A cantilever rod is loaded as shown in the figure below. If the tensile yield strength of the material is 300 MPa determine the rod diameter using (a) Maximum principal stress theory (b) Maximum...
-
Each of the following compounds can be made with a Friedel-Crafts acylation. Identify the acyl chloride and the aromatic compound you would use to produce each compound. (a) (b) O2N OCH3 H
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. In some cases, there may be more than one plausible answer. (a)...
-
In an effort to reduce alcohol consumption, the government is considering a $ 1 tax on each gallon of liquor sold (the tax is levied on producers). Suppose that the demand curve is QD = 500,000 -...
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
Use the given graph of y = ax n , where n is a nonzero integer to complete the following. (a) Is n odd or even? Is n positive or negative? (b) Is the coefficient a positive or negative? (c) Over what...
-
Which one of the following anhydrous chloride is not obtained on direct heating of its hydrated chloride? (A) BaCl2 (B) CaClz (C) MgCl2 (D) SrCl2
-
State whether you would expect each of the following properties to be identical or different for the two enantiomers of 2-pentanol. Explain. (a) Boiling point (b) Optical rotation (c) Solubility in...
-
Draw a structure for each of the following compounds in its more stable chair conformation. Explain your choice. (a) (b) CH3 CH3 CH (CH),C CHA CH, ," CH3 CH3
-
The standard free-energy difference between the two chair conformations of isopropylcyclohexane is 9.2 kJ mol-1 (2.2 kcal mol-1;. What is the ratio of concentrations of the two confirmations at 25C?
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...

Study smarter with the SolutionInn App


