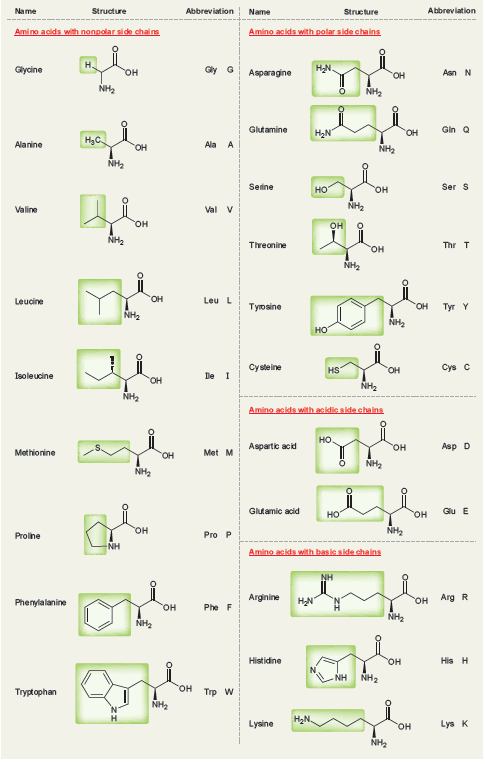
The 20 naturally occurring amino acids (See the following table) are all l amino acids, and they
Question:
Transcribed Image Text:
Abbreviation Name Structure Abbreviation Name Structure Amino acids with polar side chains Amino acids with nonpolar si de chalns н. HN. Glycine Gly G Asparagine Asn N он NH2 NH2 Gin Q Glutamine H2N Он H3C. Alanine Ala A Он NH2 NH2 Ser S Seine но он NH2 Valine Val v он он NH2 Threonine Thr T он NH3 он Leu L Leucine Tyr Y Туюaine он NH2 NH2 Cysteine Cys C HS он Isoleucine De I OH NH2 NH2 Amino acids with acidic side chains но. Aspartic acid Asp D он Methionine Met M Он NH2 NH2 Glưamic acid Giu E но он он NH2 Pro P Proline NH Amino acids with basic side chains Arg R Arginine он Phenylalanine он Phe F NH2 NH2 Histidine His H он NH2 NH он Trр W Tryptophan NH2 H2N. Lysine Lys K он NH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
When applying the CahnIngoldPrelog convention for assigning the configuration of a chiralit...View the full answer

Answered By

Niala Orodi
I am a competent and an experienced writer with impeccable research and analytical skills. I am capable of producing quality content promptly. My core specialty includes health and medical sciences, but I can competently handle a vast majority of disciplines.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify which 2 of the 20 naturally occurring amino acids are expected to have the same pI.
-
Of the 20 naturally occurring amino acids shown in the following table, identify any amino acids that exhibit the following: (a) A cyclic structure (b) An aromatic side chain (c) A side chain with a...
-
How many different pentapeptides can be constructed from the 20 naturally occurring amino acids in the following table? Abbreviation Name Structure Abbreviation Name Structure Amino acids with polar...
-
Report Format Market analysis Business - Pizza Vending Machine - Target Place : Universities 1. Target Market University Students in the UK University food 2. Potiential Customers 3. Barrier of entry...
-
Refer to the information for Concord, Inc. Requirements 1. Using variable costing, calculate the unit product cost. 2. Prepare an income statement using the contribution margin format. Use the...
-
Why is the number of protons called the atomic number?
-
Living on campus. A February 2, 2008, article in the Columbus Dispatch reported a study on the distances students lived from campus and average GPA. Here is a summary of the results: Residence Avg....
-
An electric furnace consisting of two heater sections, top and bottom, is used to heat treat a coating that is applied to both surfaces of a thin metal plate inserted midway between the heaters. The...
-
Megan is the sole shareholder of Megan's Millinery Supplies, Inc., an S corporation. Which of the following increases Megan's stock basis in the corporation
-
a. Equity turnover is sales divided by average shareholders equity. What does equity turnover measure? How is it related to return on common equity? b.Growth in earnings per share from an increase in...
-
Draw a bondline structure showing the zwitterionic form of each of the following amino acids: (a) l-Valine (b) l-Tryptophan (c) l-Glutamine (d) l-Proline
-
Draw a Fischer projection for each of the following amino acids: (a) l-Threonine (b) l-Serine (c) l-Phenylalanine (d) l-Asparagine
-
You establish a straddle on Walmart using September call and put options with a strike price of $50. The call premium is $4.25 and the put premium is $5. a. What is the most you can lose on this...
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Unless otherwise stated, assume gravitational acceleration g = 9.81 m/s and the density of water to be 1000 kg/m. Unless otherwise stated, give all numerical answers to 3 significant figures, such as...
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
Reverse the order of integration in the following integrals. cos-y S.S. 0 f(x, y) dx dy
-
On January 2, 20X3, Sheldon Bass, a professional engineer, moved from Calgary to Edmonton to commence employment with Acco Ltd., a large public corporation. Because of his new employment contract,...
-
Give the structure of the principal product(s) when each of the following alcohols reacts with (1) Na2Cr2O7/H2SO4, (2) PCC, (3) DMP, (4) NaOCl. (a) Octan-1-ol (b) Octan-3-ol (c) 4-hydroxydecanal (d)...
-
In each case, show how you would synthesize the chloride, bromide, and iodide from the corresponding alcohol. (a) 1-halobutane (halo = chloro, bromo, iodo) (b) halocyclopentane (c)...
-
Predict the major products of the following reactions, including stereochemistry where appropriate. (a) (R) - butan-2-ol + TsCI in pyridine (b) (S)-2-butyl tosylate + NaBr (c) Cyclooctanol +...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


