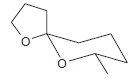
The compound below is believed to be a wasp pheromone. Draw the major product formed when this
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
H3...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The compound below is an example of a methyl ester. Methyl esters react with lithium iodide to give lithium carboxylate salts. The solvent in this example is pyridine (margin). Suggest several...
-
Identify all of the products formed when the compound below is treated with aqueous acid: N- excess H,O+
-
A material is believed to be a compound. Suppose you have several samples of this material obtained from various places around the world. Comment on what you would expect to find upon observing the...
-
Determine whether each relation defines y as a function of x. Give the domain. Identify any linear functions. y = |x|
-
Use the triangle at the right. Find the length of the missing side. Show your work. 1. a = 16, b = 63 2. b = 2.1, c = 2.9 3. A 10-foot ladder leans against a wall with its foot braced 3 feet from...
-
What is the exchange rate between Chinas currency and US dollars?
-
1. Construct Table 1 from the perspective of a seller, providing a descriptive name for each of the transactions.
-
IDX Technologies is a privately held developer of advanced security systems based in Chicago. As part of your business development strategy, in late 2008 you initiate discussions with IDXs founder...
-
the end of 2 0 2 4 . Note: Parentheses indicate a credit balance. Required: a . Prepare a worksheet to consolidate the separate 2 0 2 4 financial statements for Abbey and Bellstar. b . How would the...
-
A horizontal conveyer belt moving at a uniform speed of 1.2 M/S transports material at the rate of 100 tonnes/hr. Belt is 200 M long and driven by a motor at 1200 rpm. (a) Determine the load inertia...
-
Propose an efficient synthesis for each of the following transformations: (a) (b)
-
Propose an efficient synthesis for each of the following transformations: (a) (b) (c) OMe Meo Meo Br Br
-
Find the products by inspection. No intermediate steps should be necessary. (5 y)(7 y)
-
Alec is an employee who drives a 2021 Ford C-Max with a fair-market value of $32,000. He has been given the choice to have the fringe benefit reported on his W-2 either using the lease-value rule or...
-
What resource do most thinking and learning technologies rely on to be effective? a) data b) images c) gas d) robots
-
Financial Reporting Problem Marks and Spencer plc (M&S) The financial statements of M&S (GBR) are presented in Appendix A. The companys complete annual report, including the notes to the...
-
Totally Chemical is considering an investment decision project in which the organization expands into the trucking business. Totally Chemical wants to begin this investment decision project by buying...
-
Presented below is the balance sheet of Sandhill Corporation as of December 31, 2017. SANDHILL CORPORATION BALANCE SHEET DECEMBER 31, 2017 Goodwill (Note 2) Buildings (Note 1) Inventory Land Accounts...
-
Solve the equation. Check your answers. 3n-2 - 19n-1 + 20 = 0
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
Give the free - redical chain mechanism for the formation of ethy l bromide from ethane and bromine in the presence of light.
-
Answer Problem 8.33 for all alcohols with the forrnula C4H9OH. (a) Give the structures of all alcohols with the molecular formula C5H13OH. (b) Which of the compounds in part (a) are chiral? (c) Name...
-
Withc-rut consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) I -hexanol, 2-pentanol, tert-butr,l alcohol (b)...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


