The conversion of triglycerides into biodiesel can be achieved in the presence of either catalytic acid or
Question:

This equilibrium favors the hydroxide ions. Nevertheless, some ethoxide ions are present at equilibrium. These ethoxide ions are strong nucleophiles that can attack each ester moiety of the triglyceride according to the following mechanism.
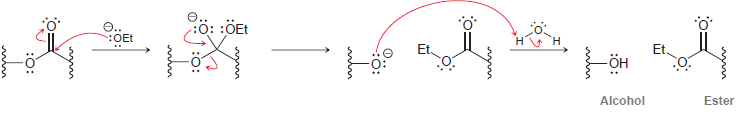
In the final step, water is deprotonated, regenerating the catalyst.
(a) Draw a mechanism for the following process.
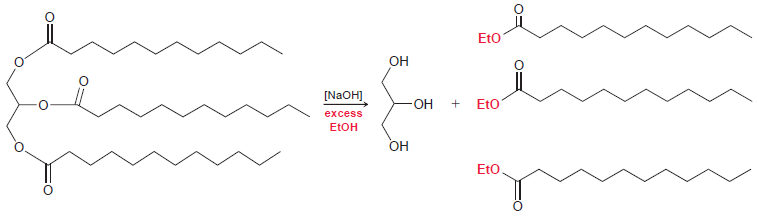
(b) When sodium hydroxide is used as a catalyst for transesterification, it is essential that only a small amount of the catalyst is present. Explain what would happen in the presence of too much sodium hydroxide.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





