The following reaction occurs via an E1 mechanistic pathway: a) What happens to the rate if the
Question:
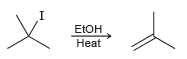
a) What happens to the rate if the concentration of tert-butyl iodide is doubled and the concentration of ethanol is tripled?
b) What happens to the rate if the concentration of tert-butyl iodide remains the same and the concentration of ethanol is doubled?
Transcribed Image Text:
ETOH Heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a Only the concentration of tertbuty...View the full answer

Answered By

Ayush Jain
Subjects in which i am expert:
Computer Science :All subjects (Eg. Networking,Database ,Operating System,Information Security,)
Programming : C. C++, Python, Java, Machine Learning,Php
Android App Development, Xamarin, VS app development
Essay Writing
Research Paper
History, Management Subjects
Mathematics :Till Graduate Level
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
The following reaction occurs via an S N 1 mechanistic pathway: (a) What happens to the rate if the concentration of tert-butyl iodide is doubled and the concentration of sodium chloride is tripled?...
-
When a mixture of methane and bromine vapor is exposed to light, the following reaction occurs slowly: CH4(g) + Br2(g) CH3Br(g) + HBr(g) Suggest a mechanism for this reaction. (Bromine vapor is deep...
-
The following reaction occurs by a general-acid-catalyzed mechanism: Propose a mechanism for this reaction. CH2 CH3 HB+
-
howwould I work these out? (a) Calculate the amount of direct materials purchased during the period. Direct materials purchased (b) Calculate the cost of goods manufactured during the period. Costs...
-
Develop an initial literature review draft. The literature review draft should be based on at least 10 to 12 peer-reviewed original sources within the past five years. Do not include book sources,...
-
Determine the domain and the range of the given functions. F(x) = 3 |x|
-
How can Google be successful in the display advertising business? What other areas of growth are likely to be pursued by Google in the future?
-
Using the information for Sarot, Inc., in SE 4 and SE 5 compute the profit margin, asset turnover, return on assets, and return on equity for 2011 and 2012. In 2010, total assets were $400,000 and...
-
Rafael a ramos received the following income during the contributory years. 1-net income filled north - 0 15,000 2-prize hypodrome - #1,000 3-TV show $ 800 4- net income of the building - $8,000 what...
-
Find two incomparable elements in these posets. a) (P({0, 1, 2}),) b) ({1, 2, 4, 6, 8}, |)
-
How does green chemistry relate to source reduction?
-
What is environmental sustainability? How are people in highly developed countries not living sustainably? How are people in developing countries not living sustainably?
-
What is the relationship between time and financial statements?
-
Drs. Draper and Keys run a partnership family medical practice in Brownsville, Texas. While the practice is profitable, both physicians are making payments on heavy debt loads for student loans that...
-
Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has only...
-
Small Sample Weights of M&M plain candies are normally distributed. Twelve M&M plain candies are randomly selected and weighed, and then the mean of this sample is calculated. Is it correct to...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
The Tokyo Olympics After watching How the Tokyo Olympics Became the Most Expensive Summer Game Ever video answer the following questions. * * The numbers can be made up . I just need help with an...
-
Simplify each expression. Assume that all variables represent nonzero real numbers. 523-3/8a-1-3 152-2 2a
-
Convert the numeral to a HinduArabic numeral. A94 12
-
The sex attractant given off by the common housefly is an alkene named muscalure. Propose a synthesis of muscalure starting from acetylene and any alkyl halides needed. What is the IUPAC name...
-
Compound A (C9H12) absorbed 3 equivalents of H2 on catalytic reduction over a palladium catalyst to give L (C9H18). On ozonolysis, compound A gave, among other things, a ketone that was identified as...
-
Hydrocarbon A has the formula C12H8. It absorbs 8 equivalents of H2 on catalytic reduction over a palladium catalyst. On ozonolysis, only two products are formed: oxalic acid (HO2CCO2H) and succinic...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


