The following two drawings are resonance structures of one compound: But the following two drawings are not
Question:
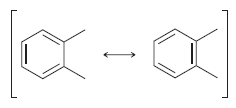
But the following two drawings are not resonance structures:
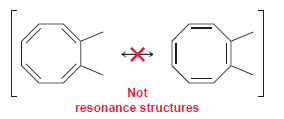
They are, in fact, two different compounds. Explain.
Transcribed Image Text:
Not resonance structures
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
Benzene does not have three CC single bond and three ...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the following compound two protons are clearly identified. Determine which of the two is more acidic. After comparing the conjugate bases, you should get stuck on the following question: Is it...
-
Draw significant resonance structures for the following compound:
-
The following three Lewis structures can be drawn for N2O: (a) Using formal charges, which of these three resonance forms is likely to be the most important? (b) The N-N bond length in N2O is 1.12...
-
Evaluate each expression if possible. -V0.49
-
Make summary for the following cases: "Hollywood on collision course with Netflix and Amazon" "Toy story: Hasbro in takeover talks with Mattel"
-
Using the truth-table definitions of the dot, the wedge, and the tilde (curl), determine which of the following statements are true: Rome is the capital of Italy Rome is the capital of Spain.
-
2. A stock currently sells for $32.00. A 6-month call option with a strike of $30.00 has a premium of $4.29, and a 6-month put with the same strike has a premium of $2.64. Assume a 4% continuously...
-
On January 1, 2012, Roosters Co. purchases equipment for $30,000 and estimates a useful life of eight years and a salvage value of $2,000. On January 1, 2014, Roosters revises the equipment's useful...
-
Peavey Enterprises purchased a depreciable asset for $26,500 on April 1. Year 1. The asset will be depreciated using the straight-line method over its four-year useful life. Assuming the asset's...
-
An investor in a 32% tax bracket and a 15%, rate on ANCG owns land that is a capital asset with a $50,000 basis and a holding period of three years. The investor who is single wishes to sell the...
-
Diphenylmethane exhibits two aromatic rings, which achieve coplanarity in the highest energy conformation. Explain. Diphenylmethane
-
Predict the major product of the following reactions. a. b. c. d. NBS Heat or light Na,Cr,0, H,SO,, H20
-
Create and test an HTML document that includes at least two images and enough text to precede the images, flow around them (one on the left and one on the right), and continue after the last image.
-
The University of Cincinnati Center for Business Analytics is an outreach center that collaborates with industry partners on applied research and continuing education in business analytics. One of...
-
For a data set of the pulse rates for a sample of adult females, the lowest pulse rate is 38 beats per minute, the mean of the listed pulse rates is x = 78.0 beats per minute, and their standard...
-
A student earned grades of A, C, B, A, and D. Those courses had these corresponding numbers of credit hours: 5, 3, 4, 3, and 2. The grading system assigns quality points to letter grades as follows:...
-
Ch 3: Forecasting: Tracking Signals, Mad, Exponential Smoothing, Control Charts Media Consultants (10 Pts). Media Consultants uses proven techniques to measure forecast accuracy and to determine when...
-
Question 2 What is the energy (in joules) of the photon absorbed by a hydrogen atom to cause a ground-state electron to move to the n = 3 energy level? Record your answer in scientific notation to 3...
-
Solve the equation. Check your answers. x-9=0
-
This problem continues the Draper Consulting, Inc., situation from Problem 12-45 of Chapter 12. In October, Draper has the following transactions related to its common shares: Oct 1 Draper...
-
Using the monomer structure in Table 5.4, draw the structure of poly(vinyl chloride) (PVC), the polymer used for the pipes in household plumbing.
-
Repeat Problem 5.27 for 1-butene
-
Draw the structure of (a) A five-carbon alkene that would give the same product of HBr addition whether peroxides are present or not. (b) A compound with the formula C6H12 that would not undergo...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


