Diphenylmethane exhibits two aromatic rings, which achieve coplanarity in the highest energy conformation. Explain. Diphenylmethane
Question:
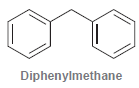
Transcribed Image Text:
Diphenylmethane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Steric hin...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Biphenyl has the following structure. (a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon? (b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number...
-
The following compound has two aromatic rings. Identify which ring is expected to be more reactive toward an electrophilic aromatic substitution reaction.
-
Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Identify the most acidic proton in each compound, and suggest a reason for the trend in...
-
If one movie ticket costs $13.50, how much will y tickets cost?
-
Research any examples of weaponized industrial network protocols cyber threats and their potential impact to Industrial network protocols. Submit your findings and be sure to include the following:...
-
Per capita income in 1960 dollars for European countries and the percent of the labor force that works in agriculture in 1960 are in table #10.1.12 ("OECD economic development," 2013). Create a...
-
1. A stock currently sells for $32.00. A 6-month call option with a strike of $35.00 has a premium of $2.27. Assuming a 4% continuously compounded risk-free rate and a 6% continuous dividend yield,...
-
Ten parts are measured three times by the same operator in a gauge capability study. The data are shown in Table 8E.9 (a) Describe the measurement error that results from the use of this gauge. (b)...
-
Slow-Gro Company makes plant food. The product is made in a single processing department Direct materials throughout the process. The company uses the FIFO method of accounting for costs. The...
-
Calculate the frequency (Hz), wavenumber (cm -1 ), and energy (J/photon and J/[mol of photons]) of visible light with a wavelength of 562 nm.
-
Would you expect the following compound to be aromatic? Justify your answer. OR N-
-
The following two drawings are resonance structures of one compound: But the following two drawings are not resonance structures: They are, in fact, two different compounds. Explain. Not resonance...
-
Explain why building up capital takes a great deal of sacrifice.
-
2. See US Debt Clock and answer the following: (Hint: Take a screenshot of the Debt Clock) (2) A. What is the current US deficit and the total federal debt? (1) B What is the net interest...
-
Q. Is GDP per capita a good measure of a society's welfare? Why or why not? (150 Words)
-
On May 3, the Happy Company wrote off the $4,300 uncollectible account of its customer, A. Johnson. The entry or entries Happy makes to record the write off of the account on May 3 is: Allowance for...
-
Who is responsible for the financial statements and maintaining effective internal control over financial reporting? Where did you find this in the annual report? What accounting rules are required...
-
8. Chad owned an office building that was destroyed in a tornado. The adjusted basis of the building at the time was $890,000. After the deductible, Chad received an insurance check for $850,000. He...
-
Solve the rational inequality 1 x + 1 >0
-
You have just begun your summer internship at Omni Instruments. The company supplies sterilized surgical instruments for physicians. To expand sales, Omni is considering paying a commission to its...
-
Consider the second propagation step of peroxide-promoted HBr addition to alkenes (Eq. 5.51b). Explain why hydrogen, and not bromine, is abstracted from HBr by the free radical reactant.
-
Consider the second propagation step of peroxide-promoted HBr addition to alkenes (Eq. 5.51b). Explain why hydrogen, and not bromine, is abstracted from HBr by the free radical reactant.
-
Using the monomer structure in Table 5.4, draw the structure of poly(vinyl chloride) (PVC), the polymer used for the pipes in household plumbing.
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


