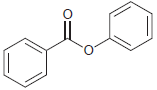
The following compound has two aromatic rings. Identify which ring is expected to be more reactive toward
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
This r...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following compound has two isomers: ClCH==CHCl One isomer has a dipole moment of 0 D, and the other has a dipole moment of 2.95 D. Propose structures for the two isomers that are consistent with...
-
The following compound has only one asymmetric carbon. Why then does it have four stereoisomers? CH CH CHCH,CH CHCH Br
-
The following compound has been found to be an inhibitor of penicillinase. The enzyme can be reactivated by hydroxylamine (NH2OH). Propose a mechanism to account for the inhibition and for the...
-
As mentioned in Section 5.6, Sainte-Venants principle will allow particular boundary conditions to be replaced by their statically equivalent resultant. For problems (b), (c), (d),and (f) in Exercise...
-
What factors should a small business manager consider when selecting advertising media?
-
Solve the given equation. 9 log(2x 1) = 3
-
3. The following information pertains to property taxes levied by Coral City for the calendar year 2016: Collections during 2016 $500,000 Expected collections during the first 60 days of 2017 100,000...
-
In 2013, Adonis Industries changed its method of valuing inventory from the average cost method to the FIFO method. At December 31, 2012, Adoniss inventories were $47.6 million (average cost)....
-
On December 31, 2018, Rhone-Metro Industries leased equipment to Western Soya Co. for a four-year period ending December 31, 2022, at which time possession of the leased asset will revert back to...
-
A company that operates 10 hours a day manufactures two products on three sequential processes. The following table summarizes the data of the problem: Determine the optimal mix of the two products....
-
For each of the following compounds, determine whether the ring is activated or deactivated, then determine the strength of activation/deactivation, and finally, determine the expected directing...
-
The following compound has four aromatic rings. Rank them in terms of increasing reactivity toward electrophilic aromatic substitution.
-
Levine Manufacturing Inc. is considering several investments. The rate on Treasury bills is currently 2.75 percent, and the expected return for the market is 12 percent. What should be the required...
-
3. Two companies (A and B) are duopolists that produce identical products. Demand for the products is given by the following demand function: P = 10,000 QA- QB - where QA and QB are the quantities...
-
Consider the following initial-value problem. f'(x) = 2ex - 6x; f(0) = 4 Integrate the function f'(x). (Remember the constant of integration.) || | f'(x)dx = Find the value of C using the condition...
-
The value chain is based on primary activities logstica Operations External logistics Marketing and sales Service and are complemented by support activities Company infrastructure is what it is,...
-
On average, both arms and hands together account for 13% of a person's mass, while the head is 7.0% and the trunk and legs account for 80%. We can model a spinning skater with her arms outstretched...
-
8. Look at the image to the right. Using the Law of Force and Acceleration, predict how acceleration would change if you changed the mass of the boy. 9. Using the same picture from #8, discuss how...
-
In Exercises 122125, determine whether each statement makes sense or does not make sense, and explain your reasoning. As production level increases, the average cost for a company to produce each...
-
CRUZ, INC. Comparative Balance Sheets December 31, 2015 CRUZ, INC. Income Statement For Year Ended December 31, 2015 Required Use the indirect method to prepare the cash provided or used from...
-
What stereochemical result would you expect if the (2s,3s)-stereoisomer of 3-bromo-2 butanol undergoes the same reaction?
-
Complete the following reaction by giving the major organic product dilute H SO CH CH CH OH (solvent)
-
Calculate the frequency of Blue light with = 4800 A
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


