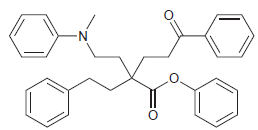
The following compound has four aromatic rings. Rank them in terms of increasing reactivity toward electrophilic aromatic
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
A B N Increasing ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following compound has two isomers: ClCH==CHCl One isomer has a dipole moment of 0 D, and the other has a dipole moment of 2.95 D. Propose structures for the two isomers that are consistent with...
-
The following compound has only one asymmetric carbon. Why then does it have four stereoisomers? CH CH CHCH,CH CHCH Br
-
The following compound has been found to be an inhibitor of penicillinase. The enzyme can be reactivated by hydroxylamine (NH2OH). Propose a mechanism to account for the inhibition and for the...
-
For problems involving composite bodies composed of two or more materials, the elasticity solution requires both boundary conditions and interface conditions between each material system. The...
-
Use a search engine to locate the most recent "E-mail Marketing Trends Survey." Using the information in it, work with a team of your classmates to select a local small business with which you are...
-
Solve the given equation. 3 log 2 (x 1) = 12
-
What amount should Coral City report for 2016 net property tax revenues? a $700,000 b $690,000 c $600,000 d $500,000
-
American Health Systems has 6,400,000 shares of stock outstanding and will report earnings of $10 million in the current year. The company is considering the issuance of 1,700,000 additional shares,...
-
Issue 4: Alan Almond Receivable Alan Almond Company (Alan Almond) owes BCE $82,000 for a computer system installation that was purchased in March of 20X3. Alan Almond has run into financial...
-
Rivera Company has several processing departments. Costs to be accounted for in the Assembly Department for November 2022 totaled $2,280,000 as follows. Production records show that 35,000 units were...
-
The following compound has two aromatic rings. Identify which ring is expected to be more reactive toward an electrophilic aromatic substitution reaction.
-
For each compound below, identify which position(s) is/are most likely to undergo an electrophilic aromatic substitution reaction. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH3 NO2 O,N. CH3 O,N NO2
-
The kitchen at a major corporation's managed service business account includes several gas and electric stoves, ovens, broilers, steamers, grills, and other appliances. On average, the kitchen serves...
-
Time (s) Velocity (cm/s or m/s) Uncertainty 0.100 -145 cm/s or 0.145 m/s +/- 0.089 m/s 0.200 -266 cm/s or 0.266 m/s +/- 0.010 m/s 0.300 -359 cm/s or 0.359 m/s +/- 0.0201 m/s 0.400 -451 cm/s or 0.451...
-
Using Technology to Generate Normal Quantile Plots. In Exercises 13-16, use the data from the indicated exercise in this section. Use software (such as Statdisk, Minitab, Excel, or StatCrunch) or a...
-
Use your understanding of work and power to answer the following questions. 1. Two physics students, Will N. Andable and Ben Pumpiniron, are in the weightlifting room. Will lifts the 100-pound...
-
Problem 2. Consider the following chemical reaction. 2H2 + O2 = 2HO Gibbs Duhem equation states that SdT - Vdp+ Nidi=0. Apply this equation for the above reaction and determine the equilibrium...
-
Part D: Exploring Pascal's Triangle 1. Fill-In the missing numbers in Pascal's Triangle. See 2. Find the sum of each row in Pascal's Triangle. Describe the pattern. 1, 2, 4, 8, 16... Power of 2n 1 1...
-
In Exercises 126129, determine whether each statement is true or false. If the statement is false, make the necessary change(s) to produce a true statement. The graph of a rational function cannot...
-
Solve the relation Exz:Solve therelation ne %3D
-
Five isomeric alkenes A-E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spectra of these five alkenes have the following key absorptions (in cm-1): Compound A: 912 (s), 994...
-
From the information in Table 12.3, predict the appearance of the molecular ion peak(s) in the mass spectrum of chloromethane. (Assume that the molecular ion is the base peak.) Table 12.3 TABLE 12.3...
-
Indicate whether the following peaks in the mass spectrum of 1-heptanol are odd-electron or even-electron ions. (a) m/z = 83 (b) m/z = 70 (c) m/z = 56 (d) m/z = 41
-
This short exercise demonstrates the similarity and the difference between two ways to acquire plant assets. (Click the icon to view the cases.) Compare the balances in all the accounts after making...
-
Balance sheet and income statement data for two affiliated companies for the current year appear below: BALANCE SHEET As at December 31, Year 6 Albeniz Bach Cash $ 40,000 $ 21,000 Receivables 92,000...
-
please reference excel cells Caroll Manufacturing company manufactures a single product. During the past three weeks, Caroll's cost accountant observed that output costs varied considerably. The...

Study smarter with the SolutionInn App


