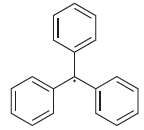
The triphenylmethyl radical was the first radical to be observed. Draw all resonance structures of this radical,
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
This radica...View the full answer

Answered By

Ashok Kumar Malhotra
Chartered Accountant - Accounting and Management Accounting for 15 years.
QuickBooks Online - Certified ProAdvisor (Advance - QuickBooks Online for 3 years.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw all resonance structures for each of the following radicals: (a) (b) (c) (d) (e)
-
Consider the structure of cyclopentadiene and then answer the following questions: (a) How many sp 3 -hybridized carbon atoms are present in the structure of cyclopentadiene? (b) Identify the most...
-
Draw the enolate ion that is formed when each of the following compounds is treated with sodium ethoxide. In each case, draw all resonance structures of the enolate ion, and predict whether a...
-
You have just been given a $454,000, which you decide to invest at an APR of 6.7 percent. If you were to withdraw $38,500 at the end of each year, starting at the end of this year, how many years...
-
Which of the following statements concerning the MM extension with growth is NOT CORRECT? a. The value of a growing tax shield is greater than the value of a constant tax shield. b. For a given D/S,...
-
Calculate the following iterated integrals. LL'xy dx) dy
-
6. U.S. Generally Accepted Accounting Principles (GAAP) and International Financial Reporting Standards (IFRS) are converging. Since this is the case, whywould amanager need to understand the...
-
Kaizer Plastics produces a variety of plastic items for packaging and distribution. One item, container #145, has had a low contribution to profits. Last year, 20,000 units of container #145 were...
-
George, owner of a successful company, thinks that some employee expenses are becoming too costly for his business, especially those that are regulated by the government, like the Employment...
-
On July 1, 2020, Academic Learning Services entered its second month of operations. On July 31, 2020, Breanne Allarie, the owner, finalized the company?s records that showed the following items. Use...
-
Calculate the pressure exerted by benzene for a molar volume of 2.00 L at 595 K using the RedlichKwong equation of state: The RedlichKwong parameters a and b for benzene are 452.0 bar dm 6 mol 2 K...
-
Use the equation C P,m C V ,m = TV m 2 / and the Data Tables to determine C V ,m for H 2 O(l) at 298 K. Calculate (C p,m C V,m )/C P,m .
-
Refer to Exercise 3. Make a histogram of the probability distribution. Describe its shape. Exercise 3. Get on the boat! A small ferry runs every half hour from one side of a large river to the other....
-
In Exercises 29 and 30, find the probabilities and indicate when the "5% guideline for cumbersome calculations" is used. 29. Medical Helicopters In a study of helicopter usage and patient survival,...
-
Introduction to Internetworking Project 1: Ctrl-Alt-Del Inc. INTRODUCTION You have accepted a contract to participate in the design of the network infrastructure of a company called Ctrl-Alt-Del Inc....
-
Construct Arguments Tell whether each statement is always true, sometimes true, or never true. Explain. a. An integer is a whole number. b. A natural number is a rational number. c. An irrational...
-
Please answer the following Questions : 1. Who are the competitors for Whole Foods? 2. Do you consider traditional supermarkets to be competitors for natural and organic supermarkets? 3. How would...
-
LNC Corp is trying to determine the effect of its inventory turnover ratio and DSO on its cash conversion. Credit sales in 2016 is $101,000, cost of goods sold will be 70% of sales and it earned a...
-
Find the difference quotient for g(x) = 4x 3 .
-
In the series connection below, what are the respective power consumptions of R, R2, and R3? R R www 4 V=6V P1-3 W; P2=3W; and P3= 3 W OP10.5 W; P2-1 W; and P3= 1.5 W P1=1.5 W; P2=1 W; and P3= 0.5 W...
-
Propose structures for amines with the following 1H NMR spectra: (a) C3H9NO (b)C4H11NO2 TMS O ppm 10 6. Chemical shift (8) TMS O ppm 10 7. 9. Chemical shift (8) Intensity Intensity -3-
-
Propose structures for compounds that show the following 1H NMR spectra. (a) C9H13N (b)C15H17N TMS 10 6 5 3 2 O ppm Chemical shift (8) TMS 3 2 O ppm 10 6 1 Chemical shift (8) Intensity Intensity-
-
?-Amino acids can be prepared by the Strecker synthesis, a two-step process in which an aldehyde is treated with ammonium cyanide followed by hydrolysis of the amino nitrile intermediate with aqueous...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


