Trans-1,3-Dichlorocyclobutane has a measurable dipole moment. Explain why the individual dipole moments of the C-Cl bonds do
Question:
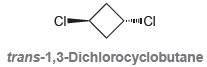
Transcribed Image Text:
CI- trans-1,3-Dichlorocyclobutane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Cyclobutene adopts a slightly puckered conformation in order to allev...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Trans-1,3-Dibromocyclobutane has a measurable dipole moment. Explain how this proves that the cyclobutane ring is not planar.
-
Which compound has the greater dipole moment? a. b. c. Cl C-C C or CI CI CI C-C or - CH3 CI CH3 CI - or - CH3
-
For each pair of compounds, predict which one has the higher molecular dipole moment, and explain your reasoning. (a) Ethyl chloride or ethyl iodide (b) 1-bromopropane or cyclopropane (c) Cis-2,...
-
Let a = (123) (45) = S, and b = (23) (14) = S5, then aba is equal to (13) (25) (135) (24) (15) (23) (123) (45)
-
What is the current membership of the European Monetary Union (EMU)? How successful has the transition to a single currency been?
-
CVP analysis is both simple and simplistic. If you want realistic analysis to underpin your decisions, look beyond CVP analysis. Do you agree? Explain.
-
In 2017, the Singapores Deputy Prime Minister Tharman Shanmugaratnam stated that the country was aiming to become a key part in the global value chain. The statement was made in the wake of the U.S....
-
The following table shows the 10 highest-paid chief executive officers of the last decade. a. Calculate the mean compensation for the 10 highest-paid chief executive officers. b. Does the mean...
-
You have $20,000 to invest in a stock portfolio. Your choices are Stock X with an expected return of 14 percent and Stock Y with an expected return of 12 percent. Your goal is to create a portfolio...
-
Consider the problem of finding the shortest path between two points on a plane that has convex polygonal obstacles as shown in Figure 3.31. This is an idealization of the problem that a robot has to...
-
In each reaction, identify the Lewis acid and the Lewis base: (a) (b) (c) F L-
-
Compound A has molecular formula C 5 H 10 . Hydroboration-oxidation of compound A produces a pair of enantiomers, compounds B and C. When treated with HBr, compound A is converted into compound D,...
-
What are the seven tests that help to establish material participation?
-
A release has been planned with 5 sprints. The team, for the sake of convenience, has decided to keep the sprint duration open. Depending on how much they commit and achieve, they decide to wrap up...
-
Task 3: Reach-truck management 3 Explain why battery-powered reach truck activities at PAPFS are unsatisfactory. Note: You should support your answer, where applicable, using relevant information...
-
Exercise 6: Black Pearl, Inc., sells a single product. The company's most recent income statement is given below. Sales $50,000 Less variable expenses Contribution margin Less fixed expenses Net...
-
Your maths problem x+3x-3
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
Use elimination to solve the system of equations, if possible. Identify the system as consistent or inconsistent. If the system is consistent, state whether the equations are dependent or...
-
B made an issue of 150,000 $1 ordinary shares at a premium of 20% the proceeds of which is received by cheque. What is the correct journal to record this? A. Bank Share capital Share premium B. Bank...
-
Explain which stereo isomer is more stable. Problems using online Three-Dimensional molecular models
-
Explain which isomer has more strain energy in the conformation shown. Problems using online Three-Dimensional molecular models
-
For these compounds, indicate whether the substituents are cis or Trans, whether they are axial or equatorial, whether the conformation shown or the other chair conformation is more stable, and...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


