Using a Gabriel synthesis, show how you would make each of the following compounds: (a) (b) (c)
Question:
(a)
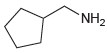
(b)
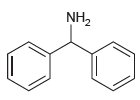
(c)
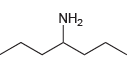
(d)
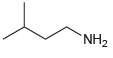
Transcribed Image Text:
`NH2 NH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a b c ...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify how you would make each of the following compounds from 1-hexanol: (a) Hexylamine (b) Heptylamine (c) Pentylamine
-
Show how you would make each compound, beginning with an alcohol of your choice. (a) (b) (c) (d) (e) (f) (g) (h) CHO CH,Br CI CH CH 1 CH C OH CH3 OTs
-
Using a Gabriel synthesis, propose an efficient synthesis for each of the following transformations. In each case, you will need to use reactions from previous chapters to convert the starting...
-
The electron affinity of oxygen is -141kJ/mol, corresponding to the reaction O (g) + e- O- (g) The lattice energy of K2O(s) is 2238kJ/mol. Use these data along with data in Appendix C and Figure 7.9...
-
On January 1, 2013, John Pierce acquired a 10 percent interest in the Saratoga and Company partnership for a cash investment of $20,000. In 2013, the partnership reported an ordinary loss of $40,000,...
-
Design a series of dashboard reports for a restaurant's business operations. (ex. sales) a. The reports should include a filter as to how to select the data b. The filter should include a location,...
-
Evaluate all base classifiers, as well as the models defined by the candidate threshold values selected in the previous exercise, using overall error rate, sensitivity, specificity, proportion of...
-
Botella Company produces plastic bottles. The unit for costing purposes is a case of 18 bottles.The following standards for producing one case of bottles have been established: Direct materials (4...
-
Remand Inc. has a beta of 1.45. If the expected market return is 10 percent and the risk-free rate is 2.5 percent, develop a comprehensive analysis of the risk and find the appropriate required...
-
Your brother has just started a new job as the Controller of an IESBA restricted audit client. You do not serve on the audit engagement. What steps must you take to ensure your independence is not...
-
The following compound cannot be prepared from an alkyl halide or a carboxylic acid using the methods described in this section. Explain why each synthesis cannot be performed. `NH2
-
Devon allocates support department costs using the direct method and estimated costs. The support department costs are budgeted at $88,000 for Department A, $63,000 for Department B, and $40,000 for...
-
The gravimetric (mass) analysis of a gaseous mixture yields CO 2 = 32%, O 2 = 56.5%, and N 2 = 11.5%. The mixture is at a pressure of 3 psia. Determine (a) The volumetric composition (b) The partial...
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
Judy and Tom agree to share the cost of an $18 pizza based on how much each ate. If Tom ate 2/3 the amount that Judy ate, how much should each pay? Tom's portion Judy's portion
-
a. What is meant by the term tax haven? b. What are the desired characteristics for a country if it expects to be used as a tax haven? c. What are the advantages leading an MNE to use a tax haven...
-
The scientists who developed Norvir stated that one of the significant interactions of the inhibitor with the enzyme is hydrogen bonding of a thiazole nitrogen (the thiazole on the right side of the...
-
Give the structures of the products expected when (t) valine and (2) proline (or other compounds indicated) react with each of the following reagents: (a) Ethanol (solvent). H2SO4 catalyst (b)...
-
In repeated attemps to synthesize the dipeptide ValLeu, aspiring peptide chemist Polly Styreen performs each of the following operations. Explain what, if anything, is wrong with procedure. The...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


