Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed
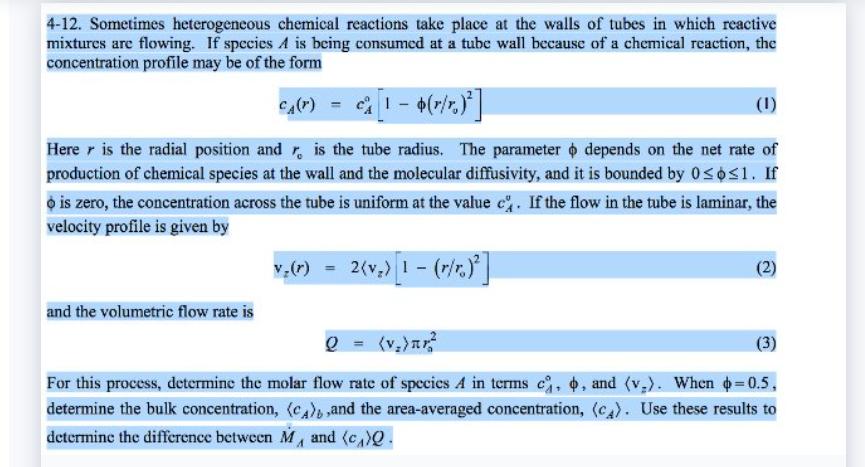
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction, the concentration profile may be of the form CA(r) (1) Here is the radial position and r is the tube radius. The parameter depends on the net rate of production of chemical species at the wall and the molecular diffusivity, and it is bounded by 061. If o is zero, the concentration across the tube is uniform at the value c. If the flow in the tube is laminar, the velocity profile is given by and the volumetric flow rate is 2 = ()? (3) and (v). When +=0.5, For this process, determine the molar flow rate of species A in terms c, determine the bulk concentration, (CA),and the area-averaged concentration, (c). Use these results to determine the difference between M, and (c)Q
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


