Using ethanol as your only source of carbon atoms, design a synthesis for the following compound:
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
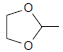
HSO4 PCC ...View the full answer

Answered By

RISHABH YADAV
I have been tutoring since more than 2 years. I teach mathematics and physics. My students have qualified a number of examinations that have gained them jobs. Some of my friends regularly keep in touch with me so as to get their doubts cleared easily and with a friendly tone. My students have never complained of being late for classes or foe not considering their issues because I always keep my tutoring and my students at topmost priority.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
Using ethanol as your only source of carbon atoms, propose a synthesis for each of the following compounds. (a) (b) OEt .
-
Using toluene as your only source of carbon atoms, show how you would prepare the following compound.
-
Using acetylene as your only source of carbon atoms, design a synthesis of trans-5- decene:
-
Simplify each expression. Assume that all variables represent nonzero real numbers. (xy-3)-2
-
You are the Certified Managerial Accountant (CMA) for Northwood Company. You have worked for the company for several years and your responsibilities include working with managers to analyze business...
-
In Exercises 13 through 18, refer to the following. Suppose a ball is thrown straight upward with an initial velocity (that is, velocity at the time of release) of 128 ft/sec and that the point at...
-
22. Repeat the previous problem calculating prices for American options instead of European. What happens?
-
Harriston Electronics builds circuit boards for a variety of applications in industrial equipment. The firm was founded in 1986 by two electrical engineers who left their jobs with General Electric...
-
Consider these transactions: ( Credit account titles are automatically indented when amount is entered. Do not indent manually. ) ( a ) Whispering Winds Restaurant accepted a Visa card in payment of...
-
PC Depot was a retail store for personal computers and hand-held calculators, selling several national brands in each product line. The store was opened in early September by Barbara Thompson, a...
-
Identify the starting materials needed to make each of the following acetals: (a) (b) (c) OEt
-
Propose an efficient synthesis for each of the following transformations: (a) (b)
-
Leasing is generally more expensive than borrowing to buy, and FASB 13 has reduced the availability of off balance sheet financing. Why then is leasing popular?
-
1. A large group of students were asked what their favorite soft drink is. Below is the probability distribution for a student chosen at random liking a particular soft drink. Drink: Choka Kola CR...
-
Task: Identify a local (within 50km of North Bay) business and answer the following questions: Name of Business: 1. Is the business independent or is it a chain? What is one advantage of this...
-
What questions would you like to ask of Cassie to better understand any factors that may be affecting Sasha at this time? Growing sunflowers It's now week 6 into the growing sunflowers project. Your...
-
n rope is fixed to a wall and attached to the block such that the rope is parallel to the surface of the wedge. The 12 points) Consider the situation in the figure where a square block (mi) sits...
-
Solve the equation. Check your answers. 0 = 9 - /1 - E/Z.X
-
Four GWU students have been selected to taste food sold by 3 different food trucks labeled as food truck A, B and C on H & 22nd Streets every Monday for 3-weeks. For each student, food trucks are...
-
Give the structure of each of the following compounds. (a) Chlorocyclopropane (b) Methylene iodide
-
Acetone (Table 8.2) has a significant dipole moment (2.7 D). Using structures, show the stabilizing interactions to be expected between acetone solvent molecules and (a) A dissolved potassium ion;...
-
Give the structure of A lecithin.
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


