Using the data in the following table, predict the sign and magnitude of ÎH° for each of
Question:
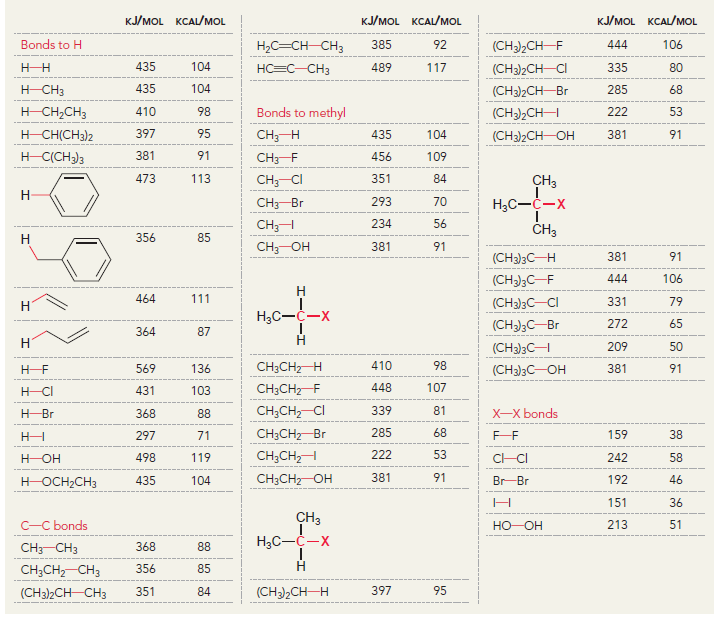
a.
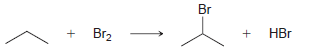
b.

c.
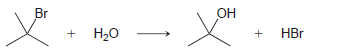
d.

Transcribed Image Text:
KJ/MOL KCAL/MOL к./мOL KCAL/MOL кJ/мOL KCAL/мOL Нас—сн сHз (CH3)2CH F (CH3)2CH CI Bonds to H 385 92 444 106 435 104 НC-С-СНз 489 117 335 80 H CH3 435 104 (CH3)2CH-Br 285 68 н сн-CHз Bonds to methyl 410 98 222 53 (CH3),CHH н СНICH)2 397 95 CHH 435 104 (CH3)2CH-OH 381 91 н ССH)з 381 91 456 109 CH3F CH3 473 113 351 84 CH; CI Н Нас—С—х 293 70 CH3-Br 234 56 CH;H CHз 356 85 Н Cн ОН 381 91 (CH)3C— Н 381 91 (CH)3C-F 444 106 Н 111 464 (CH3)3C-CI 331 79 Н Нас—с—х 272 65 (CHС—Вг 364 87 Н Н (CHд)3C— 209 50 CH3CH2 H 410 98 569 136 (CH)3с—ОН 381 91 448 107 CH3CH2 F H-CI 431 103 CH;CH,-CI 339 81 X-X bonds HBr 368 88 CH3CH2 Br 285 68 159 38 нн 297 71 222 53 CH;CH,H 498 119 C-CI 242 58 H-OH Cн CHz—ОН 381 91 435 192 46 HOCH2CH3 104 Br Br 151 36 CHз C-C bonds 213 51 но-ОН HаС —с —х CH CНз 368 88 Н CH,CH CHЗ 356 85 (CH)2CH H 351 397 95 84 (CH3)2CH CH3 Br Br, НBr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a Bonds Broken kJmol Bonds Formed kJmol H x CH CH 3 2 397 CH 3 2 CH x Br 285 Br x Br 192 H x Br 368 ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the data in the following table, calculate the pI of the following amino acids. (a) Aspartic acid (b) Leucine (c) Lysine (d) Proline THE PK,VALUES FOR TWENTY NATURALLY OCCURRING AMINO ACIDS...
-
Using the data in the following table, calculate the pI of the following amino acids: (a) l-Alanine (b) l-Asparagine (c) l-Histidine (d) l-Glutamic acid THE PK,VALUES FOR TWENTY NATURALLY OCCURRING...
-
Carbon disulfide (CS2) is a toxic, highly flammable substance. The following thermodynamic data are available for CS2(l) and CS2(g) at 298 K: (a) Draw the Lewis structure of the molecule. What do you...
-
Construct the general solution of x ' = Ax involving complex eigenfunctions and then obtain the general real solution. Describe the shapes of typical trajectories. =[ A = 3. -2 1]
-
Provide at least two examples of police corruption as it relates to drugs. Discuss some of the strategies that agencies are implementing to combat corruption in the United States or other countries.
-
Assume you are the marketing director for Perfect Seasons, a new line of cookware designed by a famous celebrity chef. In about 200 words, describe how you would use the chefs reputation to...
-
The difference between the practical capacity and the capacity based on sales expectancy is termed as (a) idle capacity (b) ideal capacity (c) return capacity (d) ordinary capacity Ans: (a)
-
Simmons Industries produced 3,000 tables last month. The standard variable manufacturing overhead (MOH) rate used by the company is $ 18 per machine hour. Each table requires 0.5 machine hours....
-
3a) Fantastic Floss Revenue for the past 5 years is listed below: Year Revenue Trend 20X120x2 20x3 20X4 20x5 $ 49,300 $ 65,20 3b) Calculate the following ratios for Fantastic Floss, Inc for the...
-
Yang Co. was organized on April 1, 2017. The company prepares quarterly financial statements. The adjusted trial balance amounts at June 30 are shown below. (a) Determine the net income for the...
-
As mentioned in problem 5.58, some molecules are chiral even though they lack a chirality center. For example, consider the following two compounds shown, and explain the source of chirality in each...
-
For each of the following processes predict the sign of ÎS for the reaction. In other words, will ÎS sys be positive (an increase in entropy) or negative (a decrease in entropy)? a. b. c....
-
Record stock transactions. (LO 1, 2, 3, 4) On the first day of the fiscal year, JKB Construction Inc. had 185,000 shares of \(\$ .50\) par common stock issued and outstanding, and the retained...
-
Will the amount of an accrual always be an exact known amount, or could it be an estimate?
-
The reorder point for SKU 303 is 102 units, while average demand during the lead time on an order for SKU 303 is 97 units. How much safety stock is implied by SKU 303's reorder point policy?
-
Find the volume of the solid obtained by rotating the region bounded by the given curves about the specified line. Sketch the region, the solid and a typical disk or washer. -2x 3. y = ex, y = 0, x =...
-
2. Given the list of scores: Score1 = [ 10, 40, 50, 54, 55, 59, 63, 65, 70, 71, 75, 77, 79, 80, 99] The one-sample T-test is used to test whether the mean of Score1 is statistically different from...
-
Find the area of the triangle having the given measurements. Round to the nearest square unit. 13) C=100, a 3 yards, b = 8 yards Use Heron's formula to find the area of the triangle. Round to the...
-
In Exercises 9194, use the graphs of f and g to evaluate each composite function. (f g)(-1) -5-4-3-2 D 3-2- y = g(x) CO y Z PI y = f(x) 2 3 4 X
-
Diamond Walker sells homemade knit scarves for $25 each at local craft shows. Her contribution margin ratio is 60%. Currently, the craft show entrance fees cost Diamond $1,500 per year. The craft...
-
For all practical purposes, the compound cyclohexa-2, 4-dien-1-one exists totally in its enol form. Write the structure of cyclohexa-2, 4-dien-1-one and of its enol form. What special factor accounts...
-
How would you use the acetoacetic ester synthesis to prepare the following?
-
How would you use the acetoacetic ester synthesis to prepare the following?
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


