For each of the following processes predict the sign of ÎS for the reaction. In other words,
Question:
a.
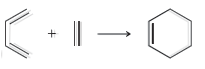
b.
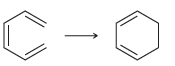
c.
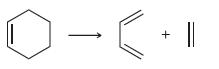
d.
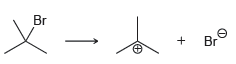
e.
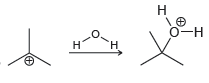
f.
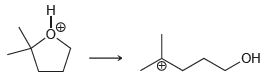
Transcribed Image Text:
||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
a S sys is expected to be negative a decrease in entropy because tw...View the full answer

Answered By

ADHITHYA NARAYANAN
I have cleared competitive exams like GATE and JEST. I also have online problem solving experiences which would come good here as well. I have experience in tutoring in Chegg and BNED platforms.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the sign of ÎS accompanying this reaction. Explain your choice.
-
The process descriptions, process flow diagrams, and technical data for processes used commercially in 38 chemical industries. For each of the following processes, draw a block-flow diagram of just...
-
For each of the following processes, indicate whether the signs of S and H are expected to be positive, negative, or about zero. (a) A solid sublimes. (b) The temperature of a sample of Co(s) is...
-
Diagonalize the matrices, if possible. The eigenvalues are as follows: (11) = 1, 2, 3; (12) = 1, 4; (13) = 5, 1; (14) = 3, 4; (15) = 3, 1; (16) = 2, 1. 3 4 6 1
-
Q1) what is the role of the project manager in the selection of the project? What criteria does the project manager use to select the project, and how are these criteria derived? Q2) List and briefly...
-
Name one important element that a company should include in its Web presence.
-
Allotment of proportions of items of cost to cost centres or cost units is known as (a) allocation (b) absorption (c) measurement (d) apportionment Ans: (d)
-
The following defined pension data of Doreen Corp. apply to the year 2014. Defined benefit obligation, 1/1/14 (before amendment) .... $560,000 Plan assets, 1/1/14 ................... 546,200 Pension...
-
Several years ago, Westmont Corporation developed a comprehensive budgeting system for planning and control purposes. While departmental supervisors have been happy with the system, the factory...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Using the data in the following table, predict the sign and magnitude of ÎH° for each of the following reactions. In each case, identify whether the reaction is expected to be endothermic...
-
At room temperature, molecules spend most of their time in lower energy conformations. In fact, there is a general tendency for any system to move toward lower energy. As another example, electrons...
-
Why is the process (as well as the result) of negotiating important to both management representatives and employee representatives? LO9
-
1. Consider the following economy: C = 3, I = 1.5, G = 2.65, T = 2, f = 0.5, d = 0.1, a = 0.8 a) Write the mathematical expression of the consumption function b) Write the mathematical expression of...
-
Question 2 (Financial statement Analysis) Following is a comparative statement of financial position for Sam's Company: Sam's Company Comparative Statement of Financial Position December 31, 2020 and...
-
Q4. Johnny's Burger is a family-run fast food joint. In addition to its famous hamburger, Johnny's Burger has just launched a new "Organic Beef burger. The owner, Johnny, would like to know if his...
-
Compute ScholarPak's break-even point in sales dollars for the year. 2. Compute the number of sales units required to earn a net income of $540,000 during the year. 3. ScholarPak's variable...
-
41-44 Find fogoh. 41. f(x)=3x-2, g(x) = sin x, 42. f(x)=|x4|, g(x) = 2, 43. f(x)=x-3, g(x) = x, h(x) = x h(x) = x h(x) = x + 2 44. f(x) = tan x, g(x) == X x-1' h(x) = x
-
In Exercises 8790, determine whether each statement makes sense or does not make sense, and explain your reasoning. When finding the inverse of a function, I interchange x and y, which reverses the...
-
Classify each of the following as direct costs or indirect costs of operating the Pediatrics ward for children at the Cleveland Clinic: a. Wi-Fi covering the entire hospital campus b. Net cost of...
-
Outline all steps in a malonic ester synthesis of each of the following: (a) pentanoic acid, (b) 2-methylpentanoic acid, (c) 4-methylpentanoic acid.
-
The antiepileptic drug valproic acid is 2-propylpentanoic acid (administered as the sodium salt). One commercial synthesis of valproic acid begins with ethyl cyanoacetate. The penultimate step of...
-
Show how you could employ enamines in syntheses of the following compounds: (a) (b) (c) (d) O C OEt
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


