Which of the following drawings is not a resonance structure for 1-nitrocyclohexene? Explain why it cannot be
Question:
a.
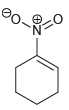
b.
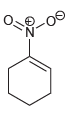
c.
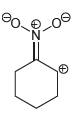
d.
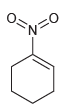
Transcribed Image Text:
0- N' Oz-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
d Is not a valid resonance structure because ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Orient each of the following drawings so that the lowest-priority group is toward the rear, and then assign R or Sconfiguration: (b) (a) (c) 2 2
-
Orient each of the following drawings so that the lowest-priority group is toward the rear, and then assign R or Sconfiguration: (b) (a) (c) 3
-
Which of the following electron configurations are not allowed? (a) 1s22s22p43s24p2; (b) 1s22s22p8p3s1; (c) 1s22s22p63523p54s24d54f1? If not allowed, explain why.
-
In Exercises find the derivative of the algebraic function. f(x) = (2x + 5x)(x 3)(x + 2)
-
On April 2nd a corporation purchased for cash 5,000 shares of its own $10 par common stock at $16 per share. They sold 3,000 of the treasury shares at $19 per share on June 15th. The remaining 2,000...
-
State the definition of continuity of a function of two variables.
-
Over which of the following costs is the management likely to have least control? (a) Advertising cost (b) Building insurance cost (c) Machine breakdown cost (d) Wage cost
-
What assumptions do developers usually make while doing the initial use case realization?
-
Question 4 - Record for Sun Company the required adjusting journal entries on December 31, 2020 for the below. 24 marks a. On Aug 1, 2020. paid $180,000 for a two-year insurance policy, b. On Oct 1,...
-
Scott Butterfield is self-employed as a CPA. He uses the cash method of accounting, and his Social Security number is 644-47-7833. His principal business code is 541211. Scott's CPA practice is...
-
What is the molecular formula for each compound in the previous problem? Previous problem a. b. c. HO
-
Identify the number of carbon atoms and hydrogen atoms in the compound below:
-
How can seasonal data be forecast with a simple bivariate linear regression model? Explain the deseasonalize-forecast-reseasonalize process. How does the material in this chapter suggest that you...
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
Match each description in Column I with the correct polynomial in Column II. Choices in Column II may be used once, more than once, or not at all. I (a) Monomial of degree 2 (b) Trinomial of degree 5...
-
Graph the following conic sections, labeling vertices, foci, directrices, and asymptotes (if they exist). Give the eccentricity of the curve. Use a graphing utility to check your work. 10 5 + 2 cos 0
-
Regarding compound J, C2HxCly, use the 1H NMR and IR data below to propose a stereochemical formula that is consistent with the data. H NMR Splitting (ppm) 6.3 3125 cm1 1625 cm1 1280 cm1 820 cm 695...
-
When dissolved in CDCl3, a compound (K) with the molecular formula C4H8O2 gives a 1H NMR spectrum that consists of a doublet at d 1.35, a singlet at d 2.15, a broad singlet at d 3.75 (1H), and a...
-
Compound T (C5H8O) has a strong IR absorption band at 1745 cm-1. The broad-band proton decoupled 13C spectrum of T shows three signals: at d 220 (C), 23 (CH2), and 38 (CH2). Propose a structure for T.
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


