Orient each of the following drawings so that the lowest-priority group is toward the rear, and then
Question:
Orient each of the following drawings so that the lowest-priority group is toward the rear, and then assign R or Sconfiguration:
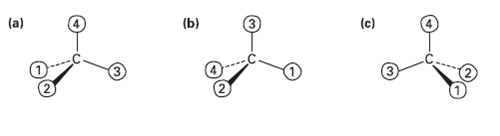
Transcribed Image Text:
(b) (a) (c) 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
a c ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Assign a priority order to each of the following sets of groups: a. -CH(CH3)2, -CH3, -H, -NH2 b. -OH, -Br, -CH3, -CH2OH c. -OCH3, -NH(CH3)2, -CH2NH2, -OH d. -CH2CH2CH3, -CH2CH3, -C(CH3)3, -CH(CH3)2
-
Assign a name for each of the following compounds. Be sure to assign the configuration of each chirality center and indicate the configuration(s) at the beginning of the name. a. b. c. Me
-
Assign IUPAC names for each of the following compounds: (a) (b) (c) (d) . CH CH3
-
Lauren Smith was relaxing after work with a colleague at a local bar. After a few drinks, she began expressing her feelings about her company's new control initiatives. It seems that as a result of...
-
Discuss the requirement that a GPS device needs information from at least three GPS satellites and why one "very good" satellite cannot do the task?
-
If your hands are wet and no towel is handy, you can remove some of the excess water by shaking them. Why does this work?
-
The ANES asks the respondent if she is married, and if she is not and there is another adult living in the household, they ask the respondent if she has a partner. Use the recoded marital status...
-
Purse Corporation owns 70 percent of Scarf Companys voting shares. On January 1, 20X3, Scarf sold bonds with a par value of $600,000 at 98. Purse purchased $400,000 par value of the bonds; the...
-
Wayne Company purchased 100% of Schuster Company on January 1, 20x1 for $800,000 when the book value of Schuster was $750,000 with the excess caused by a patent that was undervalued by $50,000. The...
-
Frequency analysis of amplitude-modulated discrete-time signal the discrete-time signal x(n) = cos2 f 1 n + cos2 f 2 n Where f 1 = 1/18 and f 2 = 5/128, modulates the amplitude of the carrier x 0 (n)...
-
What are the stereo chemical configurations of the two diastereomers of (25, 4R) -2, 4-octanediol?
-
Assign CahnIn gold-Prelog priorities to the following sets of sub-stituents: (a) H2, H)2, ICH3)3, CH2CH3 -H3)2. -C(CH3)3, -CH2CH3 () , CH2, (CH3)3 -CH=CH2, -(CH)3, () ,H , 3, H20CH3, H-H...
-
Solve the given systems of equations by Gaussian elimination. If there is an unlimited number of solutions, find two of them. 4x8y8z = 12 10x + 5y + 15z = 20 -6x - 3y 3z = 15 3x + 3y2z = 2
-
Briefly explain the difference between a k-factor model and the capital asset pricing model
-
Refer to the cost data, Picture below. Take off the square feet of wall forms and cubic yards of ready mix concrete for the walls of the elevator pit. Determine the total material and labor cost for...
-
possible Submit quiz A researcher studies water clarity at the same location in a lake on the same dates during the course of a year and repeats the measurements on the same dates 5 years later. The...
-
A liquid hydrocarbon mixture was made by adding 295 kg of benzene, 289 kg of toluene and 287 kg of p-xylene. Assume there is no change of volume upon mixing, i.e., Vmix=0 , in order to determine: 1....
-
b) Maseru Development Bank has R850 million credit with Matsieng Hydroelectric Power, with a maturity of eighteen months. The expected loss for Maseru Development Bank is R22 million, and the...
-
Describe the proper procedure for handling and serving champagne. LO.1
-
Assume today is the 21st of February. Using the information below, FT Extract, answer the following questions (parts i and ii). You work for a US company that is due to receive 250 million in June...
-
Which action causes the volume of a gas sample to increase? (a) Decreasing the pressure (at constant temperature and number of moles). (b) Decreasing the temperature (at constant pressure and number...
-
(1) 2,4 - dimethylbenzylamine (2) 2,4,6-trmethylaniline (3) NJV-dimethyl-p-methylaniline (4) 3,5-dimethyl-N-methylaniline (5) 4-ethyl-2,6-dimethylaniline 2.07 (6H, s), 2.16 (3H, s), 3.19
-
Outline a sequence of reactions that would bring about the conversion of aniline into each of the following compounds. (a) Benzyl alcohol (b) N-phenyl-2-butanamine
-
When p-aminophenol reacts with one molar equivalent of acetic anhydride, a compound acetaminophen (A, C8H9NO2) is formed that dissolves in dilute NaOH. When A is treated with one equivalent of NaOH...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


