Assign a name for each of the following compounds. Be sure to assign the configuration of each
Question:
a.
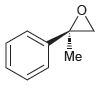
b.
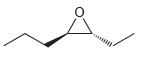
c.
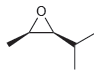
Transcribed Image Text:
Me
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
a S2phenyl1 2epoxypropane or S1me...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Assign a name for each of the following compounds. a. b. c.
-
Assign a name for each of the following compounds. a. b. c.
-
Assign a name for each of the following compounds: (a) (b) (c) (d) (e) (f) NH
-
Use the accompanying graph of y = f(x). Does exist? If it does, what is it? lim f(x)
-
Cheney Company sold a 20-ton mechanical draw press for $67,700. The old draw press cost $90,300 and had a book value of $62,000. Prepare the journal entry to record the disposition.
-
Perform the indicated operations and express the result in simplest radical form with rationalized denominators. 3x 23x - y
-
___________ report indicates whether the income is at least at the budgeted level.
-
The Finance Director of the City of Wrong Way has asked for your assistance in reviewing the Statement of Net Position that he has prepared for the year ended June 30, 20X9. The statement is shown...
-
B D E F G H 6 Jones Company was formed on December 1, 2019. The following information is available from Jones's inventory record for Product X. Units Unit Cost January 1, 2020 (beginning inventory)...
-
a. Using the relationships derived in Example Problem 7.1 and the values of the critical constants for water from Table 7.2, calculate values for the van der Waals parameters a, b, and R from z c , T...
-
Identify the reactants you would use to form a racemic mixture of each of the following epoxides: a. b. c. d. Mery Me
-
Write the numeral as a Mayan numeral. 406
-
In the following circuit, the supply voltage is 12V. Suppose VIN = +7 V, the output voltage will be, VIN VOUT R1 10 R2 10
-
Find the derivative of the function f(x)=(7x-8)". Find the derivative of the function y=- 2x x+6 Find the derivative of the function y = In Find the derivative of the function f(t): Find the...
-
Use the information below to answer questions 29 - 39. Make a table similar to examples 12 and 13 to help answer the questions. Ten persons of students at ACM are nursing students. Forty percent of...
-
Abby Industries, Inc. has the following capital structure. Type Amount Rate of Return Mortgages (debt) $25,000,000 7% Bonds (debt) 180,000,000 9% Common stock (equity) 100,000,000 10% Preferred stock...
-
Review the network of stakeholders Choose five different stakeholders and provide examples of why a project manager would need to negotiate with that stakeholder. FIGURE 10.1 Network of Stakeholders...
-
Determine whether each relation defines y as a function of x. (Solve for y first if necessary.) Give the domain. y = x - 7
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
Order the following monomers with respect to their expected reactivity toward anionic polymerization, and explain your answer: H2C = CHCH3, H2C = CHC N, H2C = CHC6H5
-
Polystyrene is produced commercially by reaction ol styrene with butyl lithium as an anionic initiator. Using resonance structures, explain how the chain-carrying intermediate is stabilized.
-
Vinylidene chloride, H2C = CCl2, does not polymerize in isotactic, syndiotactic, and atactic forms. Explain.
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


