A box divided into compartments (A) and (B) contains 14 particles in (A) and 6 in (B).
Question:
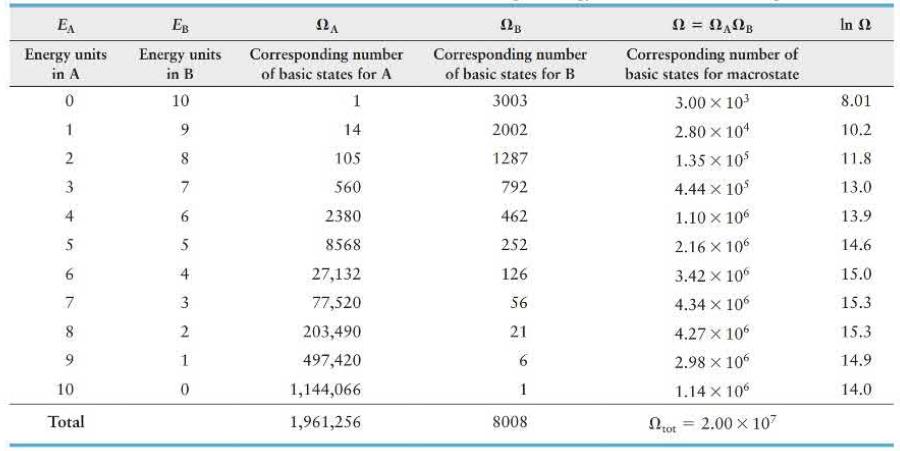
A box divided into compartments \(A\) and \(B\) contains 14 particles in \(A\) and 6 in \(B\). The separating partition allows energy exchange between compartments as the particles collide with the partition. Table 19. 2 (page 656) lists the numbers of basic states possible when 10 energy units are distributed in various combinations over the 20 particles. How much more likely is it for compartment \(A\) to contain 7 energy units (macrostate \(E_{\mathrm{A}}=7, E_{\mathrm{B}}=3\) ) than for this compartment to contain 3 energy units (macrostate \(E_{\mathrm{A}}=3, E_{\mathrm{B}}=7\) )?
Data from Table 19. 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





