Question: The K + and its excited states with masses below 1.5 GeV/c 2 are shown in Figure 3.12. Identify these mesons with states 2S +
The K+and its excited states with masses below 1.5 GeV/c2are shown in Figure 3.12. Identify these mesons with states2S+1LJof the appropriate quark€“anti quark system, specifying the value of the principal quantum number n in each case.
Figure 3.12
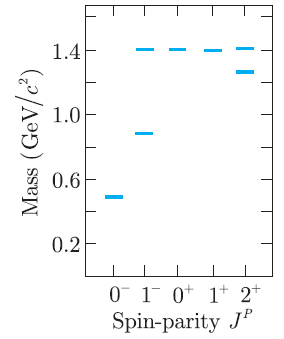
1.4 1.0- 0.6 0.2- 0 1 0* 1+ 2* Spin-parity J" Mass (GeV/c)
Step by Step Solution
3.46 Rating (162 Votes )
There are 3 Steps involved in it
The possible spectroscopic states and their J P values are the same as those ... View full answer

Get step-by-step solutions from verified subject matter experts


