a. Calculate the mass density of the H atom. b. Compare your answer with the nuclear density
Question:
b. Compare your answer with the nuclear density assuming a nuclear radius of 1.0 × 10ˆ’15m.
c. Calculate the mass density of the H atom outside of the nucleus.
Result of 20.13
The answer can be obtained using a numerical problem solver or by successive approximations. Substituting values for r/a0 in
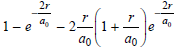
gives 0.875, 0.898, 0.905, and 0.912 for r/a0 = 2.5, 2.65, 2.7, and 2.75, respectively.
The radius of the H atom is approximately 2.65a0.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





