A correlation for methane solubility in seawater is given by the equation where is volume of
Question:
A correlation for methane solubility in seawater is given by the equation
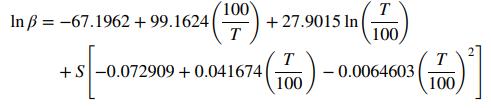
where β is volume of gas in mL at STP per unit volume (mL) of water when the partial pressure of methane is 760 mm Hg, T is temperature in Kelvin, and S is salinity in parts per thousand (ppt) by weight. At conditions of interest, the average salinity is 35 ppt, the temperature is 42°F, and the average density of seawater is 1.027 g/cm3.
(a) Estimate the mole fraction of methane in seawater for equilibrium at the given conditions. Use a mean molecular weight of 18.4 g/mol for seawater. What is the Henry’s law constant at this temperature and salinity?
(b) What does the above equation say about the effect of S on methane solubility?
(c) Use the Henry’s law constant from Part (a) to estimate methane solubility at the given temperature and salinity, but 5000 ft below the ocean surface.
Exploratory Exercise—Research and Discover
(d) At the low temperatures and high pressures associated with the depths described in Part (c), methane can combine with water to formmethane hydrates, which may affect both energy availability and the environment. Explain (i) how such behavior would influence the results in Part (c) and (ii) how dissolution of methane in seawater might affect energy availability and the environment.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




