Question: a. In this chapter, the assumption was made that the harmonic oscillator model is valid such that anharmonicity can be neglected. However, anharmonicity can be
a. In this chapter, the assumption was made that the harmonic oscillator model is valid such that anharmonicity can be neglected. However, anharmonicity can be included in the expression for vibrational energies. The energy levels for an anharmonic oscillator are given by
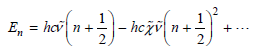
Neglecting zero-point energy, the energy levels become En = hcν̅n €“ hcχ̅ ν̅n2 +...
Using the preceding expression, demonstrate that the vibrational partition function for the anharmonic oscillator is
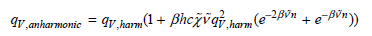
In deriving the preceding result, the following series relationship will prove useful:
b. For €“1 H2, ν̅ = 4401.2 cm and χ̅ ν̅ = 121.3 cm€“1. Use the result from part (a) to determine the percent error in qV if anharmonicity is ignored.
hc E, = hcv| n + anharmone qr ham.(1 + Bhcjva ham. (e 20in + e Bim)
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
a Performing a series expansion for the second exponential term and keepin... View full answer

Get step-by-step solutions from verified subject matter experts


