Although expressions like = d ln q/d are useful for formal manipulations in statistical thermodynamics, and for
Question:
Although expressions like <ε> = −d ln q/dβ are useful for formal manipulations in statistical thermodynamics, and for expressing thermodynamic functions in neat formulas, they are sometimes more trouble than they are worth in practical applications. When presented with a table of energy levels, it is often much more convenient to evaluate the following sums directly:
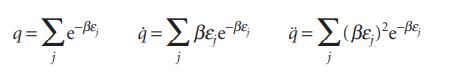
(a) Derive expressions for the internal energy, heat capacity, and entropy in terms of these three functions.
(b) Apply the technique to the calculation of the electronic contribution to the constant-volume molar heat capacity of magnesium vapour at 5000 K using the following data:
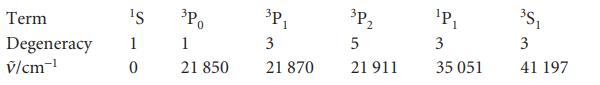
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





