An empirical expression that reproduces the viscosity of water in the range 20100 C is where
Question:
An empirical expression that reproduces the viscosity of water in the range 20–100 °C is where η20 is the viscosity at 20°C.
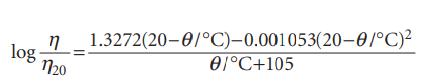
Explore (by using mathematical software) the possibility of fitting an exponential curve to this expression and hence identifying an activation energy for the viscosity.
Transcribed Image Text:
log " 1.3272(20-0/°C)-0.001053(20-0/°C}2 €1°C+105 1720
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
Answer It is possible to fit an exponential curve to the expression for the viscosity of wate...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The viscosity of water at 20 C is 1.002 cP and 0.7975 cP at 30 C. What is the energy of activation for the transport process?
-
At certain locations geothermal energy in underground water is available and used as the energy source for a power plant. Consider a supply of saturated liquid water at 150C. What is the...
-
Identifying new sources of energy has been an important business opportunity for quite some time. Given recent growth in Asia, your company is seeking the acquisition of geothermal and solar energy...
-
50 Kg of ammonium sulphate (NH4)2SO4 and 30 Kg of urea CO(NH2)2 fertilizers were applied in two equal sizes of plots A and B to enrich their nitrogen content. Show by working which plot was more...
-
A supervisor served in the Army Reserve. He reentered active status in February 2007, making it likely that he would soon be deployed. In July 2007, following receipt of an unusually low performance...
-
In Exercise, determine A - B. 4 7 2 -2 -4 9 A = 3 1 B = -2 4 -5 -1 6 4 3 6.
-
9-7. Cul es la diferencia entre sinergias de marketing y sinergias de productos en una matriz mercado-producto ?
-
Cocoaheaven processes cocoa beans into cocoa powder at a processing cost of $9,500 per batch. Cocoaheaven can sell the cocoa powder as is or it can process the cocoa powder further into either...
-
Suppose you invest $1,150 in an account paying 7% interest per year. a. What is the balance in the account after 2 years? How much of this balance corresponds to "interest on interest"? b. What is...
-
Jeffrey Vaughn, president of Frame-It Company, was just concluding a budget meeting with his senior staff. It was November of 20x0, and the group was discussing preparation of the firm's master...
-
Provide molecular interpretations for the dependencies of the diffusion constant and the viscosity on the temperature, pressure, and size of gas molecules.
-
A layer of 20.0 g of sucrose is spread uniformly over a surface of area 5.0 cm 2 and covered in water to a depth of 20 cm. What will be the molar concentration of sucrose molecules at 10 cm above the...
-
Name three reasons for using feedback control systems and at least one reason for not using them.
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 264,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Finding Confidence Intervals. In Exercises 9-16, assume that each sample is a simple random sample obtained from a population with a normal distribution. Body Temperature Data Set 5 "Body...
-
19 Part 2 of 2 1.25 points Skipped Required information Problem 6-4A & 6-5A (Algo) [The following information applies to the questions displayed below.] Gerald Utsey earned $48,400 in 2021 for a...
-
Describe equilibrium constants with words and equations. is the ratio of the concentrations of products to the concentration of reactants present in a reaction mixture when chemical equilibrium is...
-
Pronghorn Inc. acquired 20% of the outstanding common shares of Gregson Inc. on December 31, 2019. The purchase price was $1,133,000 for 51,500 shares, and is equal to 20% of Gregson's carrying...
-
A line perpendicular to another line or to a tangent line is called a normal line. Find an equation of the line perpendicular to the line that is tangent to the following curves at the given point P....
-
A random sample of 10 houses heated with natural gas in a particular area, is selected, and the amount of gas (in therms) used during the month of January is determined for each house. The resulting...
-
Sketch out a molecular orbital energy diagram for CO and place the electrons in the levels appropriate for the ground state. The AO ionization energies are O2s: 32.3 eV; O2p: 15.8 eV; C2s: 19.4 eV;...
-
Explain the difference in the appearance of the MOs in Problem P23.13 with those for HF. Based on the MO energies, do you expect LiH + to be stable? Do you expect LiH to be stable? * Li2s H1s 20 Lils...
-
Calculate the bond order in each of the following species. Predict which of the two species in the following pairs has the higher vibrational frequency: a. Li 2 or Li + 2 b. C 2 or C + 2 c. O 2 or O...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


