Explain the difference in the appearance of the MOs in Problem P23.13 with those for HF. Based
Question:
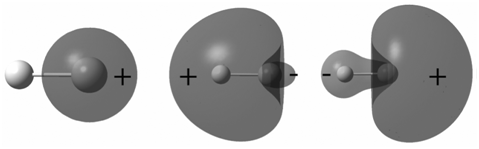
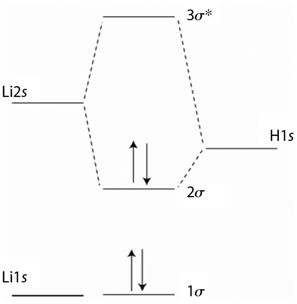
Transcribed Image Text:
За* Li2s H1s 20 Lils lo
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
The MOs on LiH are more delocalized than on HF so that ...View the full answer

Answered By

Patrick Busaka
I am a result oriented and motivated person with passion for challenges because they provide me an opportunity to grow professionally.
5.00+
38+ Reviews
58+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain the difference in the percentages of the products in these two hydroborationreactions: CH3 CH, QH CH, 1) BH3. THF 2) H,O,, NAOH CH,CH-CHCH-CH; + CH;CHCH,CHCH3 (43%) CH,CHCH=CHCH, (57%) 1)...
-
The distance between Li+ and Cl- is 257 pm in solid LiCl and 203 pm in a LiCl unit in the gas phase. Explain the difference in the bond lengths?
-
Explain the difference in the melting points of the following compounds: Only one of the two can form intramolecular hydrogen bonds? NO2 NO OH OH m.p. 115C m.p. 45C
-
On September 30, 2021, Antagonia Real Estate Limited's general ledger showed the following balances: Accounts payable Accounts receivable Advertising expenses Cash Common shares Income tax expense...
-
In Exercises 1-4, match the function with its graph. State the period of the function. [The graphs are labeled (a), (b), (c), (d), (e), and (f).] a. b. c. d. e. f. 1. y = sec 2x 2. y = tan x / 2 3. y...
-
In Problems 1922, use the method of Example 5 to find the constants A, B, and C in the indicated partial-fraction decompositions. 2x (x + 1)(x + 2)(x + 3) A x+1 + B x+2 + x+3
-
Dance/movement therapy. In cotherapy, two or more therapists lead a group. An article in the American Journal of Dance Therapy (Spring/Summer 1995) examined the use of cotherapy in dance/movement...
-
Louie Optics manufactures a underwater digital camera. The company's newest model is very popular, but it has an inventory of 5,000 old models for which there is little demand. The company is...
-
During January 2020, the first month of operations, a consulting firm had the following transactions: 2 10 points 1. Issued common stock to owners in exchange for $40,000 cash. 2. Purchased $10,000...
-
What are some of the criticisms of work measurement, in general, and time study, specifically, that have caused its popularity to wane in recent years?
-
Sketch out a molecular orbital energy diagram for CO and place the electrons in the levels appropriate for the ground state. The AO ionization energies are O2s: 32.3 eV; O2p: 15.8 eV; C2s: 19.4 eV;...
-
Calculate the bond order in each of the following species. Predict which of the two species in the following pairs has the higher vibrational frequency: a. Li 2 or Li + 2 b. C 2 or C + 2 c. O 2 or O...
-
Use the data in Table 1.7 and the Ketelaar triangle in Fig. 2.28 to predict the nature of the bonding in BeBr 2 , MgBr 2 , and BaBr 2 . Table 1.7. Figure 2.28. TABLE 1.7 Pauling Xp. Mulliken, XM, and...
-
Consider how they might directly apply to your life and work environment when answering the questions below. Competency 1: Evaluate data-driven processes and approaches of an organization's...
-
There are several website optimizer tools available to help you "increase website conversion rates." Following : Explain fully what is meant by "increase website conversion rates"; then, identify two...
-
Imagine being a human resource director for a large hotel chain. Report to management highlighting problematic diversity issues that may arise. Identify 3 challenging diversity issues (e.g., race,...
-
A study based on a sample of 4 0 0 medical school students finds that the ratio of female students is 0 . 4 8 . The school rules require that the female ratio in the school is at least 0 . 5 ? a ) (...
-
One of the many paradoxes in leadership is the challenge of encouraging a team effort while simultaneously encouraging individuals to excel. Why is this paradox a challenge for team leaders, and how...
-
In Exercises 5364, complete the square and write the equation in standard form. Then give the center and radius of each circle and graph the equation. x2 + y 7 = 0
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
Is arsenic-doped germanium a p-type or n-type semiconductor?
-
Calculate the packing fractions of (a) A primitive cubic lattice, (b) A bcc unit cell, (c) An fcc unit cell.
-
Poissons ratio for polyethylene is 0.45. What change in volume takes place when a cube of polyethylene of volume 1.0 cm 3 is subjected to a uniaxial stress that produces a strain of 1.0 per cent?
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...
Bitcoin The Emergence Of A New World Reserve Currency 1st Edition - ISBN: 979-8399741512 - Free Book

Study smarter with the SolutionInn App


