Question: An evaporationcrystallization process of the type described in Example 4.5-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The
An evaporation–crystallization process of the type described in Example 4.5-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 19.6 wt% K2SO4. The wet filter cake consists of solid K2SO4 crystals and a 40.0 wt% K2SO4 solution, in a ratio 10 kg crystals/kg solution. The filtrate, also a 40.0% solution, is recycled to join the fresh feed. Of the water fed to the evaporator, 45.0% is evaporated. The evaporator has a maximum capacity of 175 kg water evaporated/s.
(a) Assume the process is operating at maximum capacity. Draw and label a flowchart and do the degree-of-freedom analysis for the overall system, the recycle–fresh feed mixing point, the evaporator, and the crystallizer. Then write in an efficient order (minimizing simultaneous equations) the equations you would solve to determine all unknown stream variables. In each equation, circle the variable for which you would solve, but don’t do the calculations.
(b) Calculate the maximum production rate of solid K2SO4, the rate at which fresh feed must be supplied to achieve this production rate, and the ratio kg recycle/kg fresh feed.
(c) Calculate the composition and feed rate of the stream entering the crystallizer if the process is scaled to 75% of its maximum capacity.
(d) The wet filter cake is subjected to another operation after leaving the filter. Suggest what it might be. Also, list what you think the principal operating costs for this process might be.
(e) Use an equation-solving computer program to solve the equations derived in Part (a). Verify that you get the same solutions determined in Part (b).
Example 4.5-2
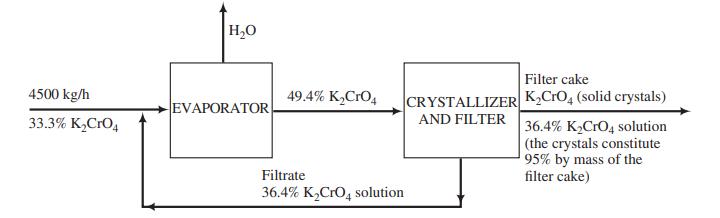
H,0 Filter cake K,CrO, (solid crystals) 4500 kg/h EVAPORATOR 49.4% K,CrO, CRYSTALLIZER 33.3% K,CrO, AND FILTER 36.4% K,CrO4 solution (the crystals constitute 95% by mass of the filter cake) Filtrate 36.4% K,CrO, solution
Step by Step Solution
3.57 Rating (168 Votes )
There are 3 Steps involved in it
To address this problem lets tackle each part stepbystep a Draw a Flowchart and DegreeofFreedom Analysis Flowchart Draw a flowchart with four main com... View full answer

Get step-by-step solutions from verified subject matter experts


