An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic
Question:
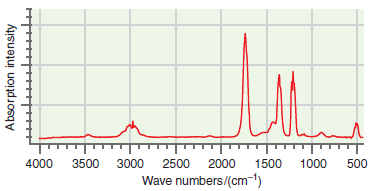
Transcribed Image Text:
T. 4000 3500 3000 2500 2000 1500 1000 500 Wave numbers/(cm-1) Absorption intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
The major peak near 1700 cm 1 is the CO stretch and the peak nea...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The market for oil-based paint is shown in the following table: Suppose the production of the paint creates a negative externality of $10 for each unit of paint, which is the cost of repairing the...
-
Use the following information to decide whether this equipment lease qualifies as an operating, sales-type, or direct financing lease to a lessor. (a) There is no transfer of ownership at the end of...
-
The part shown in the following figure is a carbon-steel segment (partial) gear. The smaller hole at the bottom is for clamping the part onto a round shaft, using a screw and a nut. Suggest a...
-
An important U.S. government organization charged with setting human resource management guidelines is O the EEOC (Equal Employment Opportunity Commission). the OSHA (Occupational Safety and Health...
-
In Exercises 1-2, use the given conditions to find the exact values of sin 2u, cos 2u, and tan 2u using the double-angle formulas. 1. sin u = 3 / 5, 3 / 2 < u < 2 2. cos u = 4 / 5, / 2 < u <
-
Determining how sensitive a stocks intrinsic value to assumptions and estimates made would be an example of _____ analytics. a. Diagnostic b. Predictive c. Descriptive d. Prescriptive
-
What two changing conditions led to the development of ERP systems? LO.1
-
Critics of absorption costing have increasingly emphasized its potential for leading to undesirable incentives for managers. Give an example.
-
QS 23-7 (Algo) Sell or process LO P2 Holmes Company has already spent $90,000 to harvest peanuts. Those peanuts can be sold as is for $69,500. Alternatively. Holmes can process further into peanut...
-
Krollon Company uses the FIFO method in its process costing system. The following data are for the most recent month of operations in one of the companys processing departments: According to the...
-
The rotational constant for 14 N 2 determined from microwave spectroscopy is 1.99824 cm 1 . Calculate the bond length in 14 N 2 to the maximum number of significant figures consistent with this...
-
Calculate the zero point energies for 1 H 19 F and 2 D 19 F. Compare the difference in the zero point energies to k B T at 298 K.
-
In Problem, evaluate the improper integrals that converge. 00 e3x dx
-
Systems thinking is all about solving problemsin organizations, world situations, and even our personal lives. But it is not just a procedure; it is a different way of approaching problems. Our...
-
How would I display the following 3 principles in an entertaining infographic? Be very specific . Principle 1: Employee Engagement and Motivation Drawing from the Human Relations Movement theory and...
-
Shown below is a cross section of tubular member which is subjected to a torque T= 5.5 kN-m. It has a length L-3.0-m and the material shear modulus G=27 GPa. Dimensions: b=150 mm, h= 100 mm and t= 8...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
In Exercises 7582, express the given function h as a composition of two functions f and g so that h(x) = (f g)(x). h(x) = |2x - 5]
-
What are some of the features of the Unified Process (UP)?
-
Consider the reaction: 2 COF 2 (g) CO 2 (g) + CF 4 (g) Kc = 2.00 In an equilibrium mixture, the concentration of COF 2 is 0.35 M and the concentration of CO 2 is 0.144 M. What is the equilibrium...
-
Consider the reaction: N 2 O 4 (g) 2 NO 2 (g) Kc = 0.36 A reaction mixture initially contains [N 2 O 4 ] = 0.100 M. Find the equilibrium concentrations of N 2 O 4 and NO 2 .
-
Consider the reaction: 2 H 2 S (g) 2 H 2 (g) + S 2 (g) Kc = 1.67 10 -7 A reaction mixture initially contains [H 2 S] = 0.010 M. Find the equilibrium concentrations of H 2 and S 2 .
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


