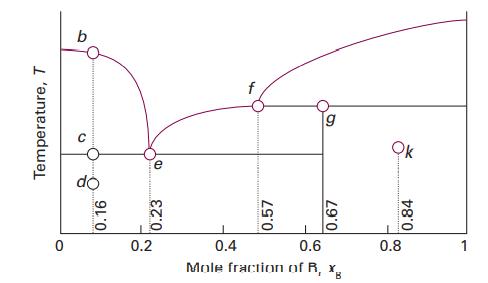
Consider the phase diagram in Fig. 5.6, which represents a solidliquid equilibrium. Label all regions of the
Question:
Consider the phase diagram in Fig. 5.6, which represents a solid–liquid equilibrium. Label all regions of the diagram according to the chemical species that exist in that region and their phases. Indicate the number of species and phases present at the points labelled b, d, e, f, g, and k. Sketch cooling curves for compositions xB =0.16, 0.23, 0.57, 0.67, and 0.84.
Data in Fig. 5.6,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:





