Figure 5.8 shows the phase diagram for the ternary system NH 4 Cl/ (NH 4 ) 2
Question:
Figure 5.8 shows the phase diagram for the ternary system NH4Cl/ (NH4)2SO4/H2O at 25 °C. Identify the number of phases present for mixtures of compositions (i) (0.2, 0.4, 0.4), (ii) (0.4, 0.4, 0.2), (iii) (0.2, 0.1, 0.7), (iv) (0.4, 0.16, 0.44). The numbers are mole fractions of the three components in the order (NH4Cl,(NH4)2SO4,H2O).
Data in Figure 5.8
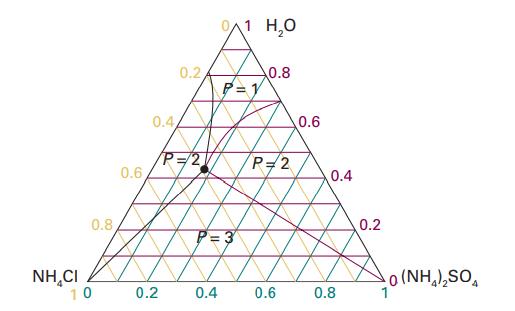
Transcribed Image Text:
NHẠCI 0.8 10 0.6 0.4 0.2 0.2/ P=2 0.4 0/1 H₂O P=1 0.8 P=2 0.6 0.6 0.4 0.8 0.2 0 (NH,),SO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (17 reviews)
At 25 C i For the mixture with mole fractions 02 04 04 there i...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Figure 5.2 shows the phase diagram for two partially miscible liquids, which can be taken to be that for water (A) and 2-methyl-1-propanol (B). Describe what will be observed when a mixture of...
-
The phase diagram for SO2 is shown here. (a) What does this diagram tell you about the enthalpy change in the reaction SO2(I) SO2(g)? (b) Calculate the equilibrium constant for this reaction at 100...
-
The phase diagram for neon is Temperature (K) Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point? (b) Over what pressure range will...
-
a. Find the probability of getting exactly 1 girl in 10 births. b. Find the probability of getting 1 or fewer girls in 10 births. c. Which probability is relevant for determining whether 1 is an...
-
On December 31, Year 1, Brown Brothers purchased machine A for $ 770,000 and machine B for $ 300,000. The machines are depreciated on the straight- line basis over 10 years with no salvage value ....
-
Determine whether some C compiler to which you have access implements the free function.
-
9. Instead of producing the tables, the company could rent its factory space for $50,000 per year. McFarland, Inc., is a merchandiser that provided the following information: Number of units sold....
-
Lion Corporation manufactures several types of accessories. For the year, the gloves and mittens line had sales of $500,000, variable expenses of $375,000, and fixed expenses of $150,000.Therefore,...
-
Required information [The following information applies to the questions displayed below.] Ferris Company began January with 8,000 units of its principal product. The cost of each unit is $9....
-
Gothic Kings Ltd. Is a 100% owned subsidiary of Hadrian Inc. Gothic has been profitable in the past but incurred a loss for the year ended December 31, 20X3. Hadrian has indicated that if Gothic...
-
Consider the phase diagram in Fig. 5.6, which represents a solidliquid equilibrium. Label all regions of the diagram according to the chemical species that exist in that region and their phases....
-
Find the relation between the standard and biological standard Gibbs energies of a reaction of the form A2B+2 H + (aq).
-
Write each expression in interval notation. Graph each interval. 6 x
-
1) Why do you believe that in recent years PE sponsors have increasingly chosen to buy debt in their distressed LBOs? 2) What are the pros and cons of this investment strategy? 3) What issues are...
-
Paper Street Soap Company Ltd conducts a business that makes luxury soaps. It operates a factory in Oyster Bay near Sydney. The factory contains a large amount of equipment that is used in the...
-
TRANSACTION ANALYSIS: Dartmouth Ties Corporation is a merchandising company that has been in operation for two years. The company sell high - end ties for men. They purchase their inventory from...
-
Using your knowledge of types of group influence and of subcultures, explain the potential impact on consumer behavior of Methodism's tightening of its ban on gay marriage and LGBTA clergy. Write in...
-
A language L over an alphabet is co-finite, if * \ Lis empty or finite. Let COFNFA = {(N) | N is a NFA accepting a co-finite language}. Show that COF NFA is decidable.
-
A wave on a rope is shown in the diagram. a. What is the wavelength of this wave? b. If the frequency of the wave is 2 Hz, what is the wave speed? 6 m E4 Diagram
-
Express mass density in kg/m3 and weight density in lb/ft3. 1. Find the mass density of a chunk of rock of mass 215 g that displaces a volume of 75.0 cm3 of water. 2. A block of wood is 55.9 in. x...
-
Consider the equilibrium in the reaction 3O 2 (g) 2O 3 (g). Assume that H o R is independent of temperature. a. Without doing a calculation, predict whether the equilibrium position will shift...
-
Why does water have several different solid phases, but only one liquid and one gaseous phase?
-
Explain how the ideal gas law can be deduced for the measurements shown in Figures 1.5 and 1.8. Figure 1.5 Figure 1.8 0.1 L 2.5 2.0 1.5 0.2 L 1.0 0.3 L 0.4 L 0.5 L 0.6 L '0.5 -200 100 0 100 200 300...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...

Study smarter with the SolutionInn App


