Deduce the relation between the pressure and mass density, , of a perfect gas of molar mass
Question:
Deduce the relation between the pressure and mass density, ρ, of a perfect gas of molar mass M. Confirm graphically, using the following data on dimethyl ether at 25°C, that perfect behaviour is reached at low pressures and find the molar mass of the gas.
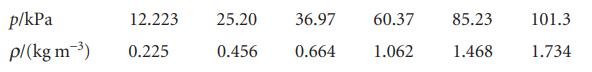
Transcribed Image Text:
p/kPa p/(kg m-³) 12.223 0.225 25.20 36.97 60.37 0.456 0.664 1.062 85.23 1.468 101.3 1.734
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)

Answered By

Jake Olson
I am an elementary education major. My favorite subject to teach is math. Last year, I was a tutor-mentor for Project for Pride in Living.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The linear mass density of a nonuniform wire under constant tension decreases gradually along the wire so that an incident wave is transmitted without reflection. The wire is uniform for x 0. In...
-
Using the following data on bond yields, calculate the change in the confidence index from last year to this year. What besides a change in confidence might explain the pattern of yield changes?...
-
Assume steady adiabatic flow of a perfect gas. Show that the energy Eq. (9.21), when plotted as a versus V, forms an ellipse. Sketch this ellipse; label the intercepts and the regions of subsonic,...
-
Describe two methods for determining the molecular mass of a polypeptide. Which is more accurate and why?
-
a. In the HJM libor model, is it a simple interest rate or a continuously compounded interest rate that follows a lognormal distribution? b. Why is this difference important (hint: relates to Blacks...
-
Is it possible for any number of hydrogen atoms to combine with just one atom of oxygen? Explain.
-
PROTECTING AGAINST CREDIT CARD FRAUD. Wyatt Scott takes pride in managing his finances carefully with great attention to detail. He recently received a phone call in which he was asked to confirm his...
-
Create a decision tree for Problem 12.
-
\ table [ [ BROOKDALE HOSPITAL ] , [ Balance Sheet ] , [ December 3 1 , 2 0 4 Brookdale Hospital hired an inexperienced controller early in 2 0 X 4 . Near the end of 2 0 X 4 , the board of directors...
-
Larry has severe vision problems and, in the past, he has claimed the additional standard deduction available to blind taxpayers. This year Larrys doctor prescribed a new type of contact lens that...
-
Explain the term partial pressure and explain why Daltons law is a limiting law.
-
A vessel of volume 22.4 dm 3 contains 2.0 mol H 2 and 1.0 mol N 2 at 273.15 K. Calculate (a) The mole fractions of each component, (b) Their partial pressures, and (c) Their total pressure.
-
Quetzal Energy Inc. issued bonds on January 1, 2014, that pay interest semi-annually on June 30 and December 31. The par value of the bonds is $240,000, the annual contract rate is 8%, and the bonds...
-
Consider: x3 + c (x 1)(x 3)(x + 1) (x + 3x + 9) (x + 2x + 5) How many partial fractions are there in the partial fraction decomposition of this function? How many unknowns (A, B, ...) must be...
-
Hand trace the following program. 1 y 0 2 for x in range (5): y = y + x 4 print ("x",x, "and y =", y) Note: You can shorten the prompts in your hand trace if you want to.
-
Determine the location using physics calculations to solve the problem. Show step by step details for how you solved the problem. I don't need an explanation explaining how to solve the problem. T By...
-
Give a brief explanation about the organization/company i.e., the products or services, number of employees, etc. Do a SWOT chart to help organize your ideas. Refer to resources in the reading for an...
-
How are organization "formal" and "informal" structures impacted in organizational change? Provide some examples. Compare and contrast Lewin's Change Model with Kotter's Change model. (Show how they...
-
Make a complete graph of the following functions on their domains or on the given interval. Use a graphing utility to check your work. f(x) = x(x - 1) e -x
-
Selected condensed data taken from a recent statement of financial position of Morino Ltd. are as follows. MORINO LTD. Statement of Financial Position (partial) Other current assets...
-
The analysis of combination differences summarized in the text considered the R and P branches. Extend the analysis to the O and S branches of a Raman spectrum.
-
Using mathematical software, elaborate on the results of Example 12A.2 by: (a) Exploring the effect of varying the wavenumbers and intensities of the three components of the radiation on the shape of...
-
Continue the development of Problem 12D.12 by using the virial expression to relate x 2 to the vibrational quantum number. Does your result imply that the rotational constant increases or decreases...
-
Given that rJ = 6.3%, rRF = 4.1%, and rM = 9.4%, determine the beta coefficient for Stock J that is consistent with equilibrium.
-
Simon Companys year-end balance sheets follow. At December 31 2017 2016 2015 Assets Cash $ 33,019 $ 37,839 $ 38,623 Accounts receivable, net 93,822 65,556 54,152 Merchandise inventory 117,963 89,253...
-
PLEASE REFER TO THE 2018 ANNUAL REPORT OF STARBUKS FOR THE YEAR FISCAL YR 2018, ENDING SEPTEMBER 30, 2018. Refer to the management discussion & analysis section and write a one page summary...

Study smarter with the SolutionInn App


