Describe the trends in Figure 17.5 that you expect to see as the quantum number n increases.
Question:
Figure 17.5
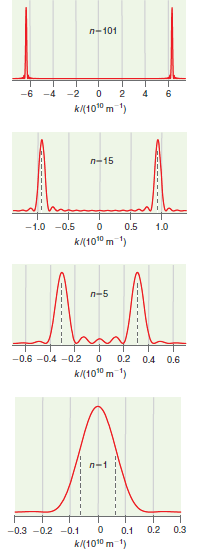
Transcribed Image Text:
n-101 -6 -4 -2 k/(1010 n-15 -1.0 -0.5 0.5 1.0 k(1010 m ) n-5 -0.6 -0.4 -0.2 0.2 0.4 0.6 k/(1010 m ') A. n-1 -0.3 -0.2 -0.1 0.2 0.3 0.1 k(1010 m 1) ---------
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
According to the correspondence principl...View the full answer

Answered By

Leah Muchiri
I am graduate in Bachelor of Actuarial Science and a certified accountant. I am also a prolific writer with six years experience in academic writing. My working principle are being timely and delivering 100% plagiarized free work. I usually present a precised solution to every work am assigned to do. Most of my student earn A++ GRADE using my precised and correct solutions.
4.90+
52+ Reviews
125+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following three compounds: CH3CHO, CH3CH2CH3, CH3CH2OH a. Describe the types of intermolecular forces that you expect to see in each. Explain how you arrived at these types. b. The heats...
-
a. Shown here are several potential Lewis electron-dot formulas for the SeO2 molecule. Are there any electron-dot formulas that you expect to give the best description? How would you describe the...
-
(a) If you spent 10 minutes repeatedly shaking and throwing down a pair of coins, would you expect to see two heads come up at least once? (b) 11 you spent an hour shaking a handful of 10 coins and...
-
On the accompanying graph, draw the consumption function C = $200 + 0.75YD. (a) At what level of income do households begin to save? Designate that point on the graph with the letter A. (b) By how...
-
Find the component form of the sum of u and v with direction angles u and v. 1. u = 4, u = 60o v = 4, v = 90o 2. u = 20, u = 45o v = 50, v = 180o
-
The following is account information listed in alphabetical order for Compu-Soft for the month ended November 30, 2023. Required Prepare a multiple-step income statement for the month ended November...
-
EXERCISE 211 Varying Plantwide Predetermined Overhead Rates LO21, LO22, LO23 Kingsport Containers Company makes a single product that is subject to wide seasonal variations in demand. The company...
-
Bunn and his wife claimed that they had an easement to enter and use the swimming pool on neighboring land. A contract between the former owners of the Bunns' property and the adjacent apartment...
-
Topsail, Inc. had the following securities in its Fair Value Investment portfolio at the end of 20x1. Stock Shares Cost / Share Alpha Corporation 200 $30.00 Fair Value I Share $28.00 Dividends were...
-
Campus Theater adjusts its accounts every month. The companys unadjusted trial balance dated August 31, 2015, is on page 176. Additional information is provided for use in preparing the companys...
-
An electron and a He atom have the same uncertainty in their speed. What can you say about the relative uncertainty in position for the two particles?
-
In this problem, we consider the calculations for Ï p and Ï x for the particle in the box shown in Figure 17.5 in more detail. In particular, we want to determine how the absolute...
-
The structure of decalin is shown below. (a) From an examination of this structure, determine its chemical formula. (b) From what aromatic hydrocarbon may decalin be produced by complete...
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
Solve each inequality. Graph the solution set, and write it using interval notation. 3 5 (1-2) - (21-7)=3 4
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
The H 2 O molecule is an asymmetric rotor with rotational constants 27.877 cm 1 , 14.512 cm 1 , and 9.285 cm 1 . Calculate the rotational partition function of the molecule at (i) 25 C, (ii) 100 C.
-
Why and when is it necessary to include a symmetry number in the calculation of a partition function?
-
How does a statistical analysis of the equilibrium constant account for the latters temperature dependence?
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


