Explain why the fluorescence and absorption groups of peaks in Figure 25.10 are shifted and show mirror
Question:
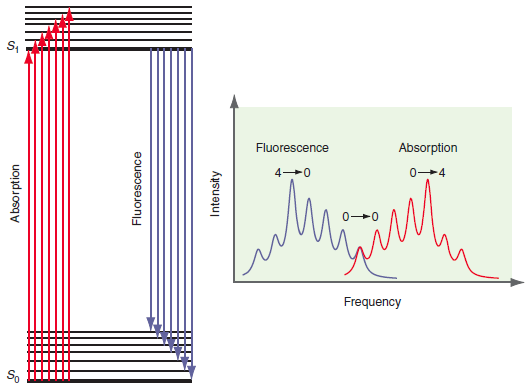
Transcribed Image Text:
Fluorescence Absorption 0-4 Frequency So Absorption Fluorescence Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
The absorption event is initiated from the n 0 vibrational level of the ground state The rela...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The fluorescence spectrum of anthracene vapour shows a series of peaks of increasing intensity with individual maxima at 440 nm, 410 nm, 390 nm, and 370 nm followed by a sharp cut-off at shorter...
-
1. G. Johnson and colleagues have analyzed the MAP kinase cascade in which MEKK2 participates in mammalian cells. By a yeast two-hybrid screen (see Chapter 7), MEKK2 was found to bind MEK5, which can...
-
What is the difference between a fluorescence excitation spectrum and a fluorescence emission spectrum? Which one resembles an absorption spectrum?
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
A guy wire runs from the ground to a cell tower. The wire is attached to the cell tower 150 feet above the ground. The angle formed between the wire and the ground is 43° (see figure). (a) How...
-
Compared to the first paragraph, the second paragraph is more A) Prescriptive. B) Despondent. C) Critical. D) Ironic.
-
Beyond simply increasing revenue, what advantages might a new business benefit from thanks to early international exposure and growth? L01
-
From a random sample of 36 business days from February 24, 2016, through February 24, 2017, the mean closing price of Apple stock was $116.16. Assume the population standard deviation is $10.27. You...
-
The following amounts were taken from the financial statements of Windsor, Inc.: 2017 2016 Total assets $801000 $1032000 Net sales 721000 662000 373000 326000 Gross profit Net income 128000 119000...
-
Jan Martinelli, a junior in college, has been seeking ways to earn extra spending money. As an active sports enthusiast. Jan plays tennis regularly at the Naples Tennis Club, where her family has a...
-
What aspect of the confocal microscope makes single-molecule spectroscopy in solutions possible?
-
The rate of fluorescence is higher than that for phosphorescence. Can you explain this fact?
-
Find expressions for the Kubo lineshape formula (t) in the limits A < >Tc.
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
In Exercises 13 through 24, compute the derivative of the given function and find the equation of the line that is tangent to its graph for the specified value x = c. f(x) = -2 X ; c = - 1
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Calculate the maximum (zero-current) potential difference of a nickelcadmium cell, and the maximum possible power output when 100 mA is drawn at 25C.
-
Derive an expression for the current density at an electrode where the rate process is diffusion-controlled and c is known. Sketch the form of j/j L as a function of c . What changes occur if anion...
-
Can magnesium be deposited on a zinc electrode from a unit activity acid solution at 25C?
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


